Abstract
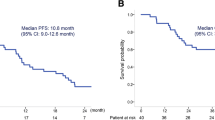
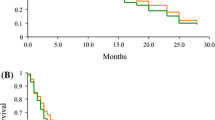
Forty-six previously untreated patients with advanced non-small cell lung cancer (NSCLC) were entered into a Hoosier Oncology Group phase II trial of daily oral etoposide 50 mg/m2/d. The dose limiting toxicity was granulocytopenia. The non-hematologic toxicity was mild, with only 19% of patients developing Grade 3 or 4 leukopenia. Two partial responses of 10 and 16 weeks duration were seen in 43 evaluable patients, for an overall response rate of 4%. We conclude that daily oral etoposide has minimal activity in advanced NSCLC, and does not improve response rates over conventional 1–5 day intravenous etoposide administration.
Similar content being viewed by others
References
Williams SD, Birch R, Einhorn LH, Irwin L, Greco FA, Loehrer PJ: Disseminated germ cell tumors: Chemotherapy with cisplatin plus bleomycin plus either vinblastine or etoposide. NEJM 316:1435–1440, 1987
Evans WK, Shepherd FA, Feld R, Osoba D, Dang P, Deboer G: VP-16 and cisplatin as first-line therapy of small-cell lung cancer. J Clin Oncol 3:1471–1477, 1985
Bender RA, Anderson T, Fisher RI, Young RC: Activity of the epipodophyllotoxin VP-16 in the treatment of combination chemotherapy-resistant non-Hodgkin's lymphoma. Am J Hematol 5:203–209, 1978
McElwain TJ, Selby P: Etoposide in combination for the treatment of Hodgkin's disease. In: Issell BF, Muggia FM, Carter SK (eds) Etoposide: Current Status and New Developments. Orlando: Academic Press, Inc., 1984, pp 293–299
Bennett JM, Lymann GH, Cassileth PA, Glick JH, Oken MM: A phase II trial of VP-16–213 in adults with refractory acute myeloid leukemia: An Eastern Cooperative Oncology Group study. Am J Clin Oncol 7:471–473, 1984
Anderson G, Peel ET, Cheong CMB, Broderick NJ: Etoposide — An effective single drug for treating bronchogenic carcinoma. J Clin Oncol 8:215–218, 1982
Chapman R, Itri L, Gralla R, Kelsen D, Casper E, Golbey R: Phase II trial of VP-16–213 in non-small cell lung cancer. Cancer Chemother Pharm 7:205–207, 1982
Slevin ML, Clark PI, Osborne RJ, et al.: A randomized trial to evaluate the effect of schedule on the activity of etoposide in small cell lung cancer. (Abstract) Proc Am Soc Clin Oncol 5:175, 1986
Stewart DJ, Nundy D, Maroun JA, Tetreault L, Prior J: Bioavailability, pharmacokinetics and clinical effects of an oral preparation of etoposide. Cancer Treat Rep 69:269–273, 1985
Cavalli F, Sonntag RW, Jungi F, Senn HS, Brunner KW: VP-16–213 monotherapy for remission induction of small cell lung cancer: A randomized trial using dosage schedules. Cancer Treat Rep 62:473–475, 1978
Hainsworth JD, Johnson DH, Frazier SR, Greco FA: Chronic daily administration of oral etoposide — a phase I trial. J Clin Oncol 7:396–401, 1989
Einhorn LH, Pennington K, McClean J: Phase II trial of daily oral VP-16 in refractory small cell lung cancer: A Hoosier Oncology Group study. Semin Oncol 17:32–35, 1990 (suppl 2)
Miller JC, Einhorn LH: Phase II study of daily oral etoposide in refractory germ cell tumors. Semin Oncol 17:36–39, 1990 (suppl 2)
Kaplan EL, Meier P: Non-parametric estimation from incomplete observations. J Am Stat Assoc 53:457–481, 1981
Brookmeyer R, Crowley J: A confidence interval for the median survival time. Biometrics 38:29–41, 1982
Author information
Authors and Affiliations
Additional information
from the Hoosier Oncology Group, the Walther Cancer Institute, Indianapolis, IN; Department of Medicine, Indiana University, Indianapolis, IN, USA
Dr. Einhorn is the Walther American Cancer Society Professor of Clinical Oncology.
Rights and permissions
About this article
Cite this article
Saxman, S., Loehrer, P.J., Logie, K. et al. Phase II trial of daily oral etoposide in patients with advanced non-small cell lung cancer. Invest New Drugs 9, 253–256 (1991). https://doi.org/10.1007/BF00176978
Issue Date:
DOI: https://doi.org/10.1007/BF00176978