Summary
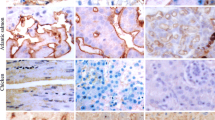
We have previously shown that the binding of the lectin wheat germ agglutinin (WGA) to developing tail buds results in a range of caudal axial defects, which were most likely due to the affinity of the lectin for sialic acid residues. In the present study, we examined the distribution and role of a sialic acid-containing glycoprotein, N-CAM, in chick tail bud development. In the early tail bud, anti N-CAM, staining was found in the medullary cord. However, there was no uptake of an antibody specific to N-CAM containing moderate to long chains of polysialic acid (5A5 monoclonal antibody). At later stages, while N-CAM localized throughout the neural tube, staining with the 5A5 antibody was restricted to the floor plate. Sub-blastodermal injection of the anti N-CAM antibody beneath the tail bud region of HH stages 13–14 embryos produced caudal axial malformations. These malformations included the presence of accessory segments of neural tube and/or notochord, and fusion between the neural tube and underlying segment of notochord. Our results suggest that N-CAM is present during the development of the secondary neuraxis from the tail bud, although the highly sialylated form of this molecule could not be visualized until relatively late stages. N-CAM probably plays a role in the normal course of tail bud development, since perturbation of the molecule with an antibody resulted in malformations. Since these malformations were similar to those we have previously reported when we treated similarly staged chick embryos with WGA, there is a possibility that the sialic acid residues recognized and bound by the lectin are those associated with the N-CAM molecule.
Similar content being viewed by others
References
Bourrillon R, Aubrey M (1989) Cell surface glycoproteins in embryonic development. Int Rev Cytol 116:257–338
Costanzo R, Watterson RL, Schoenwolf GC (1982) Evidence that secondary neurulation occurs autonomously in the chick embryo. J Exp Zool 219:233–240
Criley BB (1969) Analysis of the embryonic sources and mechanisms of development of posterior levels of chick neural tubes. J Morphol 128:465–501
Crossin KL (1989) Cell and substrate adhesion molecules in embryonic and neural development. Clin Chem 35:738–747
Dryden RJ (1980) Spina bifida in chick embryos: ultrastructure of open neural defects in the transitional region between primary and secondary modes of neural tube formation. In: Persaud TVN (ed) Neural and Behavioral Teratology, vol 4, University Park Press, Baltimore, pp 75–100
Edelman GM (1983) Cell adhesion and morphogenesis: the regulator hypothesis. Proc Natl Acad Sci USA 81:1460–1464
Griffith CM, Wiley MJ (1990a) Sialoconjugates and development of the tail bud. Development 108:479–489
Griffith CM, Wiley MJ (1990b) Distribution of cell surface glycoconjugates during chick secondary neurulation. Anat Rec 226:81–90
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–92
Hascall VC, Hascall GK (1981) Proteoglycans. In: Hay ED (ed) Cell Biology of Extracellular Matrix. Plenum Press, New York, pp 39–63
Jelinek R, Seichert V, Klika A (1969) Mechanism of morphogenesis of caudal neural tube in the chick embryo. Folia Morphol 17:355–367
Klein G, Langegger M, Goridis C, Ekblom P (1988) Neural cell adhesion molecules during embryonic induction and development of the kidney. Development 102:749–761
Kuhlmann WD, Krishan R (1981) Resin embedment of organs and postembedment localization of antigens by immunoperoxidase methods. Histochemistry 72:377–389
Rauvala H, Finne J (1979) Structural similarity of the terminal carbohydrate sequences of glycoproteins and glycolipids. FEBS Lett 97:1–8
Rutishauser U, Goridis C (1986) NCAM: the molecule and its genetics. Trends Genet 2:72–76
Rutishauser U, Watanabe M, Silver J, Troy FA, Vimr ER (1985) Specific alteration of NCAM-mediated cell adhesion by an endoneuraminidase. J Cell Biol 101:1842–1849
Rutishauser U, Acheson A, Hall AK, Mann DM, Sunshine J (1988) The neural cell adhesion molecule (NCAM) as a regulator of cell-cell interactions. Science 240:53–57
Sanders EJ (1989) The cell surface in embryogenesis and carcinogenesis. Common mechanisms. Telford Press, New Jersey
Schoenwolf GC (1979) Histological and ultrastructural observations of tail bud formation in the chick embryo. Anat Rec 193:131–148
Schoenwolf GC (1981) Morphogenetic processes involved in the remodelling of the tail region of the chick embryo. Anat Embryol 162:183–197
Schoenwolf GC (1984) Histological and ultrastructural studies of secondary neurulation in the mouse embryo. Am J Anat 169:361–376
Schoenwolf GC, DeLongo J (1980) Ultrastructure of secondary neurulation in the chick embryo. Am J Anat 158:43–63
Shedden PM, Wiley MJ (1987) Early stages of development in the caudal neural tube of the golden Syrian hamster (Mesocricetus auratus). Anat Rec 219:180–185
Smith JL, Schoenwolf GC (1989) Notochordal induction of cell wedging in the chick neural plate and its role in neural tube formation. J Exp Zool 250:49–62
van Straaten HW, Hekking JW, Thors F, Wiertz-Hoessels EL, Drukker J (1985) Induction of an additional floor plate in the neural tube. Acta Morphol Neerlanda-Scand 23:91–97
van Straaten HW, Hekking JW, Wiertz-Hoessels EJ, Thors F, Drukker J (1988) Effect of the notochord on the differentiation of a floor plate area in the neural tube of the chick embryo. Anat Embryol 177:317–324
Sunshine J, Balak K, Rutishauser U, Jacobson M (1987) Changes in neural cell adhesion molecule (NCAM) structure during vertebrate neural development. Proc Natl Acad Sci USA 84:5986–5990
Zar JH (1984) Biostatistical analysis, 2nd edn. Prentice-Hall, New Jersey
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Griffith, C.M., Wiley, M.J. N-CAM, polysialic acid and chick tail bud development. Anat Embryol 183, 205–212 (1991). https://doi.org/10.1007/BF00174400
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00174400