Abstract
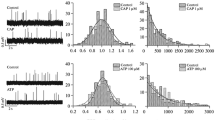
The action of the A2a-adenosine analogue, CGS 21680C, on electrically evoked [3H]acetylcholine ([3H]-ACh) release, and its interaction with forskolin (an activator of adenylate cyclase), MDL 12,330A (an irreversible inhibitor of adenylate cyclase), rolipram (an inhibitor of cyclic AMP specific phosphodiesterase), dibutyryl- (db-cAMP) and 8-bromo- (8-Br-cAMP) cyclic AMP analogues (substances that mimic intracellular actions of cyclic AMP), were investigated using rat phrenic nerve-hemidiaphragm preparations.
CGS 21680C facilitated [3H]ACh release. Forskolin (but not 1,9-dideoxy forskolin), rolipram, db-cAMP and 8-Br-cAMP also increased evoked neurotransmitter release in a concentration-dependent manner. When the evoked [3H]-ACh release that is dependent on stimulation of the adenylate cyclase/cyclic AMP transduction system was supramaximally stimulated by these compounds, CGS 21680 C (3 μmol/l) could not further increase [3H]-ACh release. Phosphodiesterase inhibition with low concentrations (⩽ 30 μmol/l) of rolipram significantly potentiated the augmenting effect of CGS 21680C (1 μmol/l) on evoked [3H]ACh release. MDL 12,330A (an irreversible inhibitor of adenylate cyclase) decreased evoked [3H]-ACh release. The irreversible blocking action of MDL 12,330A on [3H]-ACh release was overcome by by-passing cyclase activation with db-cAMP and 8-Br-cAMP, but could not be overcome with FSK or CGS 21680 C. The inhibitory effect of MDL 12,330A on evoked [3H]-ACh release was not mimicked by nifedipine.
It is concluded that the increase in [3H]-ACh release caused by CGS 21680C results from activation of an A2a-adenosine receptor positively linked to the adenylate cyclase/cyclic AMP system.
Similar content being viewed by others
References
Alousi AA, Jasper JR, Insel PA, Motulsky HJ (1991) Stoichiometry of receptor-Gs-adenylate cyclase interactions. FASEB J 5:2300–2303
Beavo JA, Reifsnyder DH (1990) Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci 11:150–155
Correia-de-Sá P, Ribeiro JA (1993) Facilitation of [3H]-ACh release by forskolin depends on A2-adenosine receptor activation. Neurosci Lett 151:21–24
Correia-de-Sá P, Ribeiro JA (1994) Potentiation by tonic A2a-adenosine receptor activation of CGRP-facilitated [3H]-ACh release from rat motor nerve endings. Br J Pharmacol 111:582–588
Correia-de-Sá P, Sebastiào AM, Ribeiro JA (1991) Inhibitory and excitatory effects of adenosine receptor agonists on evoked transmitter release from phrenic nerve endings of the rat. Br J Pharmacol 103:1614–1620
Correia-de-Sá P, Timóteo MA, Ribeiro JA (1992) Presynaptic A2 adenosine receptors of the motor nerve endings of the rat are coupled to adenylate cyclase/cyclic AMP transducing system. Br J Pharmacol 105:312P
Cushing DF, Brown GL, Sabouni MH, Mustafa SF (1991) Adenosine receptor-mediated coronary artery relaxation and cyclic nucleotide production. Am J Physiol 261:H343-H348
Daly JW, Padgett W, Seamon KB (1982) Activation of cAMP-generating systems in brain membranes and slices by the diterpene forskolin: augmentation of receptor-mediated responses. J Neurochem 38:532–544
Davis CW (1984) Assessment of selective inhibition of rat cerebral cortical calcium-independent and calcium-dependent phosphodiesterases in crude extracts using deoxycyclic AMP and potassium ion. Biochim Biophys Acta 797:354–362
Ginsborg BL, Jenkinson DH (1976) Transmission of impulses from nerve to muscle. In: Zaimis E (ed) Neuromuscular Junction. Springer, Berlin Heidelberg New York, pp 229–364
Goldberg AL, Singer JJ (1969) Evidence for a role of cyclic AMP in neuromuscular transmission. Proc Natl Acad Sci USA 64:134–141
Grupp G, Grupp IL, Johnson CL, Matlib MA, Ronslin W, Schwartz A, Wallick ET, Wang T, Wisler P (1980) Effect of RMI 12,330A, a new inhibitor of adenylate cyclase on myocardial function and subcellular activity. Br J Pharmacol 70:429–442
Guellaen G, Mahn JI, Mavier P, Berthelot P, Hanouve J (1977) RMI 12,330A, an inhibitor of adenylate cyclase in rat liver. Biochim Biophys Acta 484:465–475
Henion WF, Sutherland EW, Posternak TH (1967) Effects of derivatives of adenosine 3′,5′-phosphate on liver slices and intact animals. Biochim Biophys Acta 148:106–113
Hunt NH, Evans T (1980) RMI 12,330A, an inhibitor of nucleotide phosphodiesterases and adenylate cyclase in kidney preparations. Biochim Biophys Acta 613:499–506
Hutchinson AJ, Wells RL, Oci HH, Ghai GR, Zimmerman MB, Williams M (1989) CGS 21680C, an A2 selective adenosine receptor agonist with preferential hypotensive activity. J Pharmacol Exp Ther 251:47–55
Katz B (1969) The release of neural transmitter substances. Liverpool University Press, Liverpool
Laurenza A, Sutkowski EMcH, Seamon KB (1989) Forskolin: a specific stimulator of adenylate cyclase or a diterpene with multiple sites of action. Trends Pharmacol Sci 10:442–447
Markstein R, Digger K, Marshall NR, Starke K (1984) Forskolin and the release of noradrenaline in cerebrocortical slices. Naunyn Schmiedebergs Arch Pharmacol 325:17–24
Miller RJ (1987) Multiple calcium channels and neuronal function. Science 235:46–52
Premont RT, Jacobowitz O, Iyengar R (1992) Lowered responsiveness of the catalyst of adenylate cyclase to stimulation by Gs in heterologous desensitization: A role for cyclic adenosine 3′,5′-monophosphate-dependent phosphorylation. Endocrinology 131:2774–2784
Rampe D, Triggle DJ (1990) New ligands for L-type Ca2+ channels. Trends Pharmacol Sci 11:112–115
Rasmussen H, Barrett PQ (1984) Calcium messenger systems: an integrated view. Physiol Rev 64:938–984
Ribeiro JA, Sá-Almeida AM (1984) Excitatory effects of dibutyryl cyclic AMP, noradrenaline and theophylline on 45Ca uptake by synaptosomes from rat brain. Arch Int Pharmacodyn Ther 2:215–231
Schepp W, Soll AH, Walsh JH (1990) Dual modulation by adenosine of gastrin release from canine G-cells in primary culture. Am J Physiol 259:556–563
Schwabe U, Miyake M, Ohga Y, Daly JW (1976) 4-(3-cyclopentyloxy-4-methoxyphenyl)-2-pyrrolidone (ZK 62711): a potent inhibitor of adenosine cyclic 3′,5′-monophosphate in homogenates and tissue slices from rat brain. Mol Pharmacol 12:900–910
Schwabe U, Trost T (1980) Characterization of adenosine receptors in rat brain by (−)[3H]-N6-phenylisopropyladenosine. Naunyn Schmiedebergs Arch Pharmacol 313:179–187
Seamon KB, Padgett W Daly JW (1981) Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci USA 78:3363–3367
Sebastião AM, Ribeiro JA (1990) Interactions between adenosine and phorbol esters or lithium at the frog neuromuscular junction. Br J Pharmacol 100:55–62
Sheldon RL, Eichberg J (1993) An A2 adenosine receptor mechanism modulates phosphoinositide metabolism in peripheral nerve. J Neurochem 61 [Suppl]:S78C
Siggins GR (1978) Electrophysiological assessment of mononucleotides and nucleosides as first and second messengers in the nervous system. In: Karlin A, Tennyson VM, Vogel HJ (eds) Neuronal information transfer. Academic Press, New York, pp 339–355
Simon LN, Shuman DA, Robins RK (1973) The chemistry and biological properties of nucleotides related to nucleoside 3′,5′-cyclic phosphates. In: Greengard P, Robinson GA (eds) Advances in cyclic nucleotides research. Raven Press New York, pp 225–353
Strada SJ, Martin MW, Thompson WJ (1984) General properties of multiple molecular forms of cyclic nucleotides phosphodiesterases in the nervous system. Adv Cyclic Nucleotide Protein Phosphorylation Res 16:13–29
Takagi T, Kojima M, Nagata M, Kuromi H (1970) On the site of action of hemicholinium-3 at the rat phrenic nerve-diaphragm preparation with special references to its multiple presynaptic actions. Neuropharmacology 9:359–367
Wessler I (1989) Control of transmitter release from the motor nerve by presynaptic nicotinic and muscarinic autoreceptors. Trends Pharmacol Sci 10:110–114
Wessler I, Kilbinger H (1986) Release of [3H]-acetylcholine from a modified rat phrenic nerve-hemidiaphragm preparation. Naunyn Schmiedebergs Arch Pharmacol 334:357–364
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Correia-de-Sá, P., Ribeiro, J.A. Evidence that the presynaptic A2a-adenosine receptor of the rat motor nerve endings is positively coupled to adenylate cyclase. Naunyn-Schmiedeberg's Arch Pharmacol 350, 514–522 (1994). https://doi.org/10.1007/BF00173021
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00173021