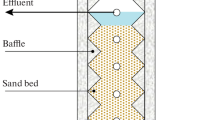
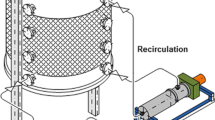
Summary
A laboratory-scale fluidized-bed reactor was inoculated with a syntrophically propionate-degrading co-culture. After 24 days of batch operation with propionate and acetate in the medium, the reactor was operated for 8 months with propionate as the sole substrate. The loading rate was 8.5 kg chemical oxygen demand/m3 ·day, yielding a maximal gas production of 4.5 l/l·day at a removal efficiency of 94–99%. The degradation was not inhibited by up to 85mm propionate in pulse experiments, but short-time changes in redox potential above — 300 mV led to a drop in the propionate degradation rate. While Methanocorpusculum sp. and Methanospirillum sp. adhered to the sand in the early phase of the start-up, the consortium in the mature biofilm consisted of Desulfobulbus sp., Methanothrix soehngenii and species of at least three different genera of hydrogenotrophic methanogens. A syntrophic relationship between the sulphate-reducing Desulfobulbus sp. and hydrogenotrophic and acetotrophic methanogens is discussed.
Similar content being viewed by others
References
Barredo MS, Evison LM (1991) Effect of propionate toxicity on methanogen-enriched sludge, Methanobrevibacter smithii, and Methanospirillum hungatii at different pH values. Appl Environ Microbiol 57:1764–1769
Boone DR, Bryant MP (1980) Propionate-degrading bacterium Syntrophobacter wolinii sp. nov. gen. nov., from methanogenic ecosystems. Appl Environ Microbiol 40:626–632
Boone DR, Xun L (1987) Effects of pH, temperature, and nutrients on propionate degradation by a methanogenic enrichment culture. Appl Environ Microbiol 53:1589–1592
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bryant MP, Campbell LL, Reddy CA, Crabill MR (1977) Growth of Desulfovibrio in lactate or ethanol media low in sulfate in association with H2-utilizing methanogenic bacteria. Appl Environ Microbiol 33:1162–1169
Chynoweth DP, Mah RA (1971) Volatile acid formation in sludge digestion. Adv Chem Ser 105:41–54
Ferry JG, Smith PH, Wolfe RS (1974) Methanospirillum, a new genus of methanogenic bacteria, and characterization of Methanospirillum hungatii sp. nov. Int J Syst Bacteriol 24:465–469
Fukuzaki S, Nishio N, Shobayashi M, Nagai S (1990) Inhibition of the fermentation of propionate to methane by hydrogen, acetate, and propionate. Appl Environ Microbiol 56:719–723
Gorris LGM (1987) Analysis of methanogenic populations in anaerobic digesters. PhD thesis, University of Nijmegen, The Netherlands
Gorris LGM, Deursen JMA van, Drift C van der, Vogels GD (1989a) Inhibition of propionate degradation by acetate in methanogenic fluidized bed reactors. Biotechnol Lett 11:61–66
Gorris LGM, Deursen JMA van, Drift C van der, Vogels GD (1989b) Biofilm development in laboratory methanogenic fluidized bed reactors. Biotechnol Bioeng 33:687–693
Guyot J-P, Brauman A (1986) Methane production from formate by syntrophic association of Methanobacterium bryantii and Desulfovibrio vulgaris JJ. Appl Environ Microbiol 52:1436–1437
Heyes RH, Hall RJ (1983) Kinetics of two subgroups of propionate-using organisms in anaerobic digestion. Appl Environ Microbiol 46:710–715
Hobson PN, Bousfield S, Summers R (1974) Anaerobic digestion of organic matter. Crit Rev Environ Control 4:131–191
Houwen FP, Dijkema C, Schoenmakers CHH, Stams AJM, Zehnder AJB (1987) 13C-NMR study of propionate degradation by a methanogenic coculture. FEMS Microbiol Lett 41:269–274
Houwen FP, Plokker J, Stams AJM, Zehnder AJB (1990) Enzymatic evidence for involvement of the methylmalonyl-CoA pathway in propionate oxidation by Syntrophobacter wolinii. Arch Microbiol 155:52–55
Huser BA, Wuhrmann K, Zehnder AJB (1982) Methanothrix soehngenii gen nov. spec. nov., a new acetotrophic nonhydrogen oxidizing methane bacterium. Arch Microbiol 132:1–9
Jee HS, Nishio N, Nagai S (1987) Influence of redox potential on biomethanation of H2 and CO2 by Methanobacterium thermoautotrophicum in Eh-stat batch cultures. J Gen Appl Microbiol 33:401–408
Jee HS, Mano T, Nishio N, Nagai S (1988) Influence of redox potential on methanation of methanol by Methanosarcina barkeri in Eh-stat batch culture. J Ferment Technol 66:123–126
Kaspar HF, Wuhrmann K (1978) Kinetic parameters and relative turnovers of some important catabolic reactions in digesting sludge. Appl Environ Microbiol 36:1–7
Kjaergaard L (1977) The redox potential: its use and control in biotechnology. Adv Biochem Eng 7:131–150
Koch M, Dolfing J, Wuhrmann K, Zehnder AJB (1983) Pathways of propionate degradation by enriched methanogenic cultures. Appl Environ Microbiol 45:1411–1414
Kremer DR, Hansen TA (1988) Pathway of propionate degradation in Desulfobulbus propionicus. FEMS Microbiol Lett 49:273–277
Lang E, Lang H (1972) Spezifische Farbreaktion zum direkten Nachweis der Ameisensäure. Z Anal Chem 260:8–10
Mackie RI, Bryant MP (1981) Metabolic activity of fatty acid-oxidizing bacteria and the contribution of acetate, propionate, butyrate and CO2 to methanogenesis in cattle waste at 40 and 60° C. Appl Environ Microbiol 41:1363–1373
McCarty PL (1971) Energetics and kinetics of anaerobic treatment. Adv Chem Ser 105:91–107
McCarty PL, Smith DP (1986) Anaerobic wastewater treatment. Environ Sci Technol 20:1200–1206
McInerney MJ, Bryant MP (1981) Anaerobic degradation of lactate by syntrophic association of Methanosarcina barkeri and Desulfovibrio species and effect of H2 on acetate degradation. Appl Environ Microbiol 41:346–354
Newport PJ, Nedwell DB (1988) The mechanisms of inhibition of Desulfovibrio and Desulfotomaculum species by selenate and molybdate. J Appl Bacteriol 65:419–423
Sahm H (1984) Anaerobic waste water treatment. Adv Biochem Eng Biotechnol 29:83–115
Samain E, Dubourguier HC, Albagnac G (1984) Isolation and characterization of Desulfobulbus elongatus sp. nov. from a mesophilic industrial digester. Syst Appl Microbiol 5:391–401
Schink B (1985) Mechanisms and kinetics of succinate and propionate degradation in anoxic freshwater sediments and sewage sludge. J Gen Microbiol 131:643–650
Shapiro M, Switzenbaum MS (1984) Initial anaerobic biofilm development. Biotechnol Lett 6:729–734
Smith DP, McCarty PL (1989a) Energetic and rate effects on methanogenesis of ethanol and propionate in perturbed CSTRs. Biotechnol Bioeng 34:39–54
Smith DP, McCarty PL (1989b) Reduced product formation following perturbation of ethanol- and propionate-fed methanogenic CSTRs. Biotechnol Bioeng 34:885–895
Stams AJM, Kremer DR, Nicolay K, Weenk GH, Hansen TA (1984) Pathway pf propionate formation in Desulfobulbus propionicus. Arch Microbiol 139:167–173
Switzenbaum MS, Giraldo-Gomez E, Hickey RF (1990) Monitoring of the anaerobic methane fermentation process. Enzyme Microb Technol 12:722–730
Tanimoto Y, Tasaki M, Okamura K, Yamaguchi M, Minami K (1989) Screening growth inhibitors of sulfate-reducing bacteria and their effects on methane fermentation. J Ferment Bioeng 68:353–359
Tatton MJ, Archer DB, Powell GE, Parker ML (1989) Methanogenesis from ethanol by defined mixed continuous cultures. Appl Environ Microbiol 55:440–445
Ten Brummeler E, HulshoffPol LW, Dolfing J, Lettinga G, Zehnder AJB (1985) Methanogenesis in an upflow anaerobic sludge blanket reactor at pH 6 on an acetate-propionate mixture. Appl Environ Microbiol 49:1472–1477
Terho TT, Hartiala K (1971) Method for determination of sulfate content of glycosaminoglycans. Anal Biochem 41:471–476
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Thauer RK, Möller-Zinkhan D, Spormann AM (1989) Biochemistry of acetate catabolism in anaerobic chemotrophic bacteria. Annu Rev Microbiol 43:43–67
Verrier D, Mortier B, Albagnac G (1987) Initial adhesion of methanogenic bacteria to polymers. Biotechnol Lett 9:735–740
Waffenschmidt S, Jaenicke L (1987) Assay of reducing sugars in the nanomole range with 2,2'-bicinchoninate. Anal Biochem 165:337–340
Wegener WS, Reeves HC, Rabin R, Ajl SJ (1968) Alternative pathways of metabolism of short-chain fatty acids. Bacteriol Rev 32:1–26
Widdel F, Pfennig N (1982) Studies on dissimilatory sulfate fate-reducing bacteria that decompose fatty acids. II. Incomplete oxidation of propionate by Desulfobulbus propionicus gen. nov., sp. nov. Arch Microbiol 131:360–365
Wolin EA, Wolin MJ, Wolfe RS (1963) Formation of methane by bacterial extracts. J Biol Chem 238:2882–2886
Wollersheim R, Selz A, Heppner B, Diekmann H (1989) Enrichment of acetate-, propionate-, and butyrate-degrading co-cultures from the biofilm of an anaerobic fluidized bed reactor. Appl Microbiol Biotechnol 31:425–429
Yadav VK, Archer DB (1988) Specific inhibition of sulphate-reducing bacteria in methanogenic co-culture. Lett Appl Microbiol 7:165–168
Zehnder AJB, Wuhrmann K (1976) Titanium (III) citrate as a nontoxic oxidation-reduction buffering system for the culture of obligate anaerobes. Science 194:1165–1166
Zeikus JG, Henning DL (1977) Methanobacterium arbophilicum sp. nov. an obligate anaerobe isolated from wetwood of living trees. Antonie van Leeuwenhoek 41:543–552
Zellner G, Winter J (1987) Analysis of a highly efficient methanogenic consortium producing biogas from whey. Syst Appl Microbiol 9:284–292
Zellner G, Stackebrandt E, Messner P, Tindall BJ, Conway de Macario E, Kneifel H, Sletyr UB, Winter J (1989) Methanocorpusculaceae fam. nov., represented by Methanocorpusculum parvum, Methanocorpusculum sinense spec. nov. and Methanocorpusculum bavaricum spec. nov. Arch Microbiol 151:381–390
Zellner G, Sleytr UB, Messner P, Kneifel H, Winter J (1990) Methanogenium liminatans spec. nov., a new coccoid, mesophilic mathanogen able to oxidize secondary alcohols. Arch Microbiol 153:287–293
Zellner G, Geveke M, Conway de Macario E, Diekmann H (1991a) Population dynamics of biofilm development during start-up of a butyrate-degrading fluidized-bed reactor. Appl Microbiol Biotechnol 36:404–409
Zellner G, Geveke M, Diekmann H (1991b) Start-up and operation of a fluidized-bed reactor oxidizing butyrate by a defined syntrophic population. Biotechnol Lett 13:687–691
Author information
Authors and Affiliations
Additional information
Offprint requests to: G. Zellner
Rights and permissions
About this article
Cite this article
Heppner, B., Zellner, G. & Diekmann, H. Start-up and operation of a propionate-degrading fluidized-bed reactor. Appl Microbiol Biotechnol 36, 810–816 (1992). https://doi.org/10.1007/BF00172200
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00172200