Abstract
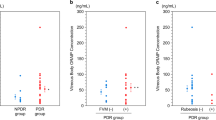
• Background: Retinal pigment epithelium cells and activated phagocytes are believed to be involved in the pathogenesis of proliferative vitreoretinopathy (PVR). Both cell types are capable of producing oxygen free radicals and other molecules with a high oxidative potential which can lead to a propagation of oxidative damage. It was the aim of this study to investigate whether products of oxidative reactions are detectable in the vitreous body of patients suffering from PVR. • Methods: In vitreous aspirates of patients vitrectomized because of PVR (n = 27), macular pucker (n = 9), or other reasons (controls, n = 31), the following parameters were determined: lipid peroxides (LPO), determined as malondialdehyde-like substances (MDA) and as thiobarbituric acid-reactive substances (TBARS), and myeloperoxidase activity (MPO). • Results: Compared with the controls, both LPO levels and MPO activities were significantly elevated in the vitreous of patients suffering from PVR. Vitreous of patients with macular pucker did not reveal any significant differences from controls in the parameters analyzed. • Conclusion: Our results suggest that both oxygen free radicals and inflammation-related reactions participate in the process of PVR. Oxidative tissue damage is obviously not involved in the pathogenesis of macular pucker.
Similar content being viewed by others
References
Asada K, Takahashi M, Tanaka K, Nakano Y (1977) In: Hayashi O, Asada K (eds) Biochemical and medical aspects of active oxygen. Japan Science Society Press, Tokyo, pp 45–63
Augustin AJ, Lutz J (1991) Intestinal, hepatic and renal production of thiobarbituric acid reactive substances and myeloperoxidase activity after temporary aortic occlusion and reperfusion. Life Sci 49:961–968
Augustin AJ, Breipohl W, Böker T, Wegener A (1992) Evidence for the prevention of oxidative tissue damage in the inner eye by vitamin E and vitamin C. Germ J Ophthalmol 1:394–398
Borgeat P, Samuelsson B (1979) Transformation of arachidonic acid by rabbit polymorphonuclear leukocytes. J Biol Chem 254:2643–2646
Bradley PP, Priebat DA, Christensen RD, Rothstein G (1982) Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78:206–209
Cariou R, Harosseau JL, Tobelem G (1988) Inhibition of human endothelial cell proliferation by heparin and steroids. Cell Biol Int Rep 12:1037–1047
Clark R, Stone RD, Leung DYK, Silver I, Hohn DC, Hunt TK (1976) Role of macrophages in wound healing. Surg Forum 27:16–18
Esterbauer H, Lang J, Zadravec S, Slater TF (1984) Detection of malondialdehyde by high-performance liquid chromatography. Methods Enzymol 105:319–328
Gaudric A, Glacet-Bernard A, Falquerho L, Barritault D, Coscas G (1989) Transforming growth factor beta in vitreous from patients with epiretinal proliferation. In: Heimann K, Wiedemann P (eds) Proliferative vitreoretinopathy. Kaden, Heidelberg, pp 118–119
Glaser BM, Connor TB, Roberts AB, Sporn MB, Danielpour D, Dart LL, Michels RG, Bustros S de, Enger C (1989) Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. In: Heimann K, Wiedemann P (eds) Proliferative vitreoretinopathy. Kaden, Heidelberg, pp 120–127
Goldstein IM, Roos D, Kaplan HB, Weissmann G (1975)Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest 56:1155–1163
Granger DN, Rutili G, McCord JM (1981) Superoxide radicals in feline intestinal ischemia. Gastroenterology 81:22–29
Hartmann, JR, Robinson JA, Gunnar RM (1977) Chemotactic activity in the coronary sinus after experimental myocardial infarction: effects of pharmacologic intervention on ischemic injury. Am J Cardiol 40:550–555
Heffernan JT, Futterman S, Kalina RE (1978) Dexamethasone inhibition of experimental endothelial cell proliferation in retinal venules. Invest Ophthalmol Vis Sci 17:565–568
Hiscott PS, Grierson I, McLeod D (1984) Retinal pigment epithelial cells in epiretinal membranes: an immunohistological study. Br J Ophthalmol 68:708–715
Hui YN, Goodnight R, Sorgente N, Ryan SJ (1989) Fibrovascular proliferation and retinal detachment after intravitreal injection of activated macrophages in the rabbit eye. Am J Ophthalmol 108:176–184
Iyer GYN, Islam DMF, Quastel JH (1961) Biochemical aspects of phagocytosis. Nature 192:535–541
Jerdan JA, Pepose JS, Michels R, Hayashi H, Bustros S de, Sebag M, Glaser BM (1989) Proliferative vitreoretinopathy membranes. An immunohistochemical study. Ophthalmology 96:801–810
Kampik A, Kenyon KR, Michels RG, Green WR, Cruz ZC de la (1981) Epiretinal and vitreous membranes. Comparative study of 56 cases. Arch Ophthalmol 99:1445–1454
Leibovich SJ, Ross R (1976) A macrophage-dependant factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol 84:501–514
Leibovich SJ, Wiseman DM (1988) Macrophages, wound repair and angiogenesis. Prog Clin Biol Res 266:131–145
Lewis GD, Campell WB, Johnson AR (1986) Inhibition of prostaglandin synthesis by glucocorticosteroids in human endothelial cells. Endocrinology 119:62–69
Lucchesi BR, Mullane KM (1986) Leukocytes and ischemia-induced myocardial injury. Ann Rev Pharmacol Toxicol 26:201–204
Machemer R, Laqua FH (1975) Pigment epithelium proliferation in retinal detachment (massive periretinal proliferation). Am J Ophthalmol 80:1–23
Mandelcorn MS, Machemer R, Fineberg E, Hersch SB (1975) Proliferation and metaplasia of intravitreal pigment epithelial cell autotransplants. Am J Ophthalmol 80:227–237
Michels RG (1989) Macular pucker. In: Ryan SJ (ed) Retina. Mosby, St. Louis, pp 419–430
Naveh N, Weissman C (1990) Corticosteroid treatment of laser retinal damage affects prostaglandin E2 response. Invest Ophthalmol Vis Sci 31:9–13
Newsome DA, Dobard EP, Liles MR, Oliver PD (1990) Human retinal epithelium contains two distinct species of superoxide dismutase. Invest Ophthalmol Vis Sci 31:2508–2513
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid. Anal Biochem 95:351–358
Reed PW (1969) Glutathione and the hexose monophosphate shunt in phagocytizing and hydrogen peroxide-treated rat leukocytes. J Biol Chem 2459–2464
Sbarra JA, Karnowsky ML (1959) The biochemical basis of phagocytosis. J Biol Chem 234:1355–1362
Schacterle GR, Pollack RL (1973) A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem 51:654–655
Thérasse J, Lemonnier F (1987) Determination of plasma lipoperoxides by high-performance liquid chromatography. J Chromatogr 413:237–241
Weller M, Wiedemann P, Moter H, Heimann K (1989) Transferrin and transferrin receptor expression in intraocular proliferative disease. APAAP-immunolabeling of retinal membranes and ELISA for vitreal transferrin. Graefe's Arch Clin Exp Ophthalmol 227:281–286
Weller M, Clausen R, Heimann K, Wiedemann P (1990) Iron-binding proteins in the human vitreous: lactoferrin and transferrin in health and in proliferative intraocular disorders. Ophthalmic Res 22:194–200
Weller M, Wiedeman P, Heiman K (1990) Proliferative vitreoretinopathy — is it anything more than wound healing at the wrong place? Int Ophthalmol 14:105–117
Wolter JR (1960) The macrophages of the human vitreous body. Am J Ophthalmol 49:1185–1193
Author information
Authors and Affiliations
Additional information
This study was presented in part at the first annual meeting of ECORA, 4–6 October, 1993 in Bonn
Rights and permissions
About this article
Cite this article
Böker, T., Augustin, A.J., Breipohl, W. et al. Increased lipid peroxide level and myeloperoxidase activity in the vitreous of patients suffering from proliferative vitreoretinopathy. Graefe's Arch Clin Exp Ophthalmol 232, 652–656 (1994). https://doi.org/10.1007/BF00171379
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00171379