Abstract
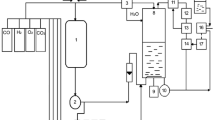
Methylobacterium extorquens ATCC 55366 was successfully cultivated at very high cell densities in a fed-batch fermentation system using methanol as a sole carbon and energy source and a completely minimal culture medium for the production of poly-β-hydroxybutyrate (PHB). Cell biomass levels were between 100 g/l and 115 g/l (dry weight) and cells contained between 40% and 46% PHB on a dry-weight basis. PHB with higher molecular mass values than previously reported for methylotrophic bacteria was obtained under certain conditions. Shake-flask and fermentor experiments showed the importance of adjusting the mineral composition of the medium for improved biomass production and higher growth rates. High-cell-density cultures were obtained without the need for oxygen-enriched air; once the oxygen transfer capacity of the fermentor was reached, methanol was thereafter added in proportion to the amount of available dissolved oxygen, thus preventing oxygen limitation. Controlling the methanol concentration at a very low level (less than 0.01 g/l), during the PHB production phase, led not only to prevention of oxygen limitation but also to the production of very high-molecular-mass PHB, in the 900–1800 kDa range. Biomass yields relative to the total methanol consumed were in the range 0.29–0.33 g/g, whereas PHB yields were in the range 0.09–0.12 g/g. During the active period of PHB synthesis, PHB yields relative to the total methanol consumed were between 0.2 g/g and 0.22 g/g. M. extorquens ATCC 55366 appears to be a promising organism for industrial PHB production.
Similar content being viewed by others

References
Abbate M, Martuscelli E, Ragosta G, Scarinzi G (1991) Tensile properties and impact behaviour of poly(d(−)3-hydroxybutyrate)/rubber blends. J Mater Sci 26:1119–1125
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54:450–472
Anderson AJ, Williams DR, Taidi B, Dawes EA, Ewing DF (1992) Studies on copolyester synthesis by Rhodococcus ruber and factors influencing the molecular mass of polyhydroxybutyrate accumulated by Methylobacterium extorquens and Alcaligenes eutrophus. FEMS Microbiol Rev 103:93–102
ATCC (1985) Gherna R, Nierman W, Pienta P (eds) Catalogue of bacteria, phages and rDNA vectors, 16th edn. ATCC, Rockville, Md
Berger É, Ramsay BA, Ramsay JA, Chavarie C, Braunegg G (1989) PHB recovery by hypochlorite digestion of non-PHB biomass. Biotechnol Tech 3:227–232
Bourque D, Ouelette B, André G, Groleau D (1992a) Production of poly-β-hydroxybutyrate from methanol: characterization of a new isolate of Methylobacterium extorquens. Appl Microbiol Biotechnol 37:7–12
Bourque D, Pomerleau Y, Groleau D (1992b) High cell density production of PHB using methanol in a fed-batch system. In: Vert M, Feijen J, Albertsson A, Scott G, Chiellini E (eds) Proceedings of the Second International Workshop on Biodegradable Polymers and Plastics 1991, Montpellier, France. (Royal Society of Chemistry, Cambridge, UK)
Brandl H, Gross RA, Lenz RW, Fuller CR (1990) Plastics from bacteria and for bacteria: poly(β-hydroxyalkanoates) as natural, biocompatible, and biodegradable polyesters. In: A. Fiechter (ed) Advances in biochemical engineering/biotechnology, vol 41. Springer, Berlin Heidelberg New York, pp 77–93
Byrom D (1987) Polymer synthesis by microorganisms: technology and economics. Trends Biotechnol 5:246–250
Byrom D (1991) Miscellaneous biomaterials. In: Byrom D (ed) Biomaterials: novel materials from biological sources. Stockton, New York, pp 333–359
Choi JH, Kim JH, Daniel M, Lebeault JM (1989) Optimization of growth medium and poly-β-hydroxybutyric acid production from methanol in Methylobacterium organophilum. Kor J Appl Microbiol Bioeng 17:392–396
Daniel M, Choi JH, Kim JH, Lebeault JM (1992) Effect of nutrient deficiency on accumulation and relative molecular weight of poly-β-hydroxybutyric acid by methylophic bacterium, Pseudomonas 135. Appl Microbiol Biotechnol 37:702–706
Doronina NV, Ostafin M, Trotsenko YA (1992) Methylobacterium extorquens — a new facultative methylotroph forming poly-β-hydroxybutyrate. Microbiology 61:481–485
Govorukhina NI, Trotsenko YA (1991) Poly-β-hydroxybutyrate contents of methylotrophic bacteria with different routes for methanol assimilation. Appl Biochem Microbiol 27:80–83
Groleau D, Bourque D, Pormerleau Y (1994) Methylobacterium extorquens microorganism useful for the preparation of poly-β-hydroxybutyric acid polymers. United States patent 5 302 525
Haywood GW, Anderson AJ, Dawes EA (1989) A survey of the accumulation of novel polyhydroxyalkanoates by bacteria. Biotechnol Lett 11:471–476
Hilger U, Sattler K, Littkowsky U (1991) Studies on the growth-associated accumulation of poly-β-hydroxybutyric acid with Methylobacterium rhodesianum Z. Zentralbl Mikrobiol 146:83–88
Holmes PA (1988) Biologically produced (B)-3-hydroxyalkanoate polymers and copolymers. In: Bassett DC (ed) Developments in crystalline polymers, vol 2, Elsevier, London, pp 1–65
Lafferty RM, Korsatko B, Korsatko W (1988) Microbial production of poly-β-hydroxybutyric acid. In: Rehm HJ, Reed G (eds) Biotechnology, vol 6B. VCH, Weinheim, pp 135–176
Large PJ, Bamforth CW (1988) Methylotrophy and biotechnology. Longman, Scientific & Technical, Burnt Mill, Harlow
Reymann W, Koch M, Albrecht P (1990) Manufacture of containers from an oriented biopolymer. Tech Mitt Krupp 2:113–118
Steinbüchel A (1991) Polyhydroxyalkanoic acids. In: Byrom D (ed) Biomaterials: novel materials from biological sources. Stockton, New York, pp 123–213
Suzuki T, Yamane T, Shimizu S (1986a) Mass production of poly-β-hydroxybutyric acid by fully automatic fed-batch culture of methylotroph. Appl Microbiol Biotechnol 23:322–329
Suzuki T, Yamane T, Shimizu S (1986b) Kinetics and effect of nitrogen source feeding on production of poly-β-hydroxybutyric acid by fed-batch culture. Appl Microbiol Biotechnol 24:366–369
Suzuki T, Yamane T, Shimizu S (1986c) Mass production of poly-β-hydroxybutyric acid by fed-batch culture with controlled carbon/nitrogen feeding. Appl Microbiol Biotechnol 24:370–374
Suzuki T, Deguchi H, Yamane T, Shimizu S, Gekko K (1988) Control of molecular weight of poly-β-hydroxybutyric acid production in fed-batch culture of Protomonas extorquens. Appl Microbiol Biotechnol 27:487–491
Taidi B, Anderson AJ, Dawes EA, Byrom D (1994) Effect of carbon source and concentration on the molecular mass of poly(3-hydroxybutyrate) produced by Methylobacterium extorquens and Alcaligenes eutrophus. Appl Microbiol Biotechnol 40:786–790
Toennies G, Gallant D (1949) The relation between photometric turbidity and bacterial concentration (bacterimetric studies IV). Growth 13:7–20
Trotsenko YA, Doronina NV, Sokolov AP, Ostafin M (1993) Synthesis of polyhydroxybutyrate by methylotrophic bacteria. FEMS Microbiol Rev 103:393–394
Ueda S, Matsumoto S, Takagi A, Yamane T (1992) Synthesis of poly(3-hydroxybutyrat-co-3-hydroxyvalerate) from methanol and n-amyl alcohol by the methylotrophic bacteria Paracoccus denitrificans and Methylobacterium extorquens. Appl Environ Microbiol 58:3574–3579
Valentin HE, Steinbüchel A (1993) Cloning and characterization of the Methylobacterium extorquens polyhydroxyalkanoic-acid-synthase structural gene. Appl Microbiol Biotechnol 39:309–317
Yamane T (1992) Cultivation engineering of microbial bioplastics production. FEMS Microbiol Rev 103:257–264
Yasin M, Tighe BJ (1993) Strategies for the design of biodegradable polymer systems: manipulation of polyhydroxybutyrate-based materials. Plast Rubber Composites Process Appl 19:15–27
Zhao S, Fan C, Hu X, Chen J, Feng H (1993) The microbial production of polyhydroxybutyrate from methanol. Appl Biochem Biotechnol 39/40:191–199
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bourque, D., Pomerleau, Y. & Groleau, D. High-cell-density production of poly-β-hydroxybutyrate (PHB) from methanol by Methylobacterium extorquens: production of high-molecular-mass PHB. Appl Microbiol Biotechnol 44, 367–376 (1995). https://doi.org/10.1007/BF00169931
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00169931