Summary
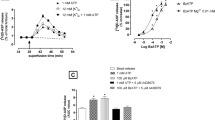
Adenosine agonists produce antinociception when injected directly onto the spinal cord of rats and mice. One mechanism to account for this effect could be inhibition of neurotransmitter release from nociceptive sensory neurons. Consequently, we studied whether these agents could inhibit the potassium stimulated release of one such transmitter, substance P, from rat spinal cord slices. A 2 cm section of lumbar spinal cord was dissected from male Sprague-Dawley rats, chopped into 0.5 × 0.5 mm sections and perfused at 37°C with a modified Krebs bicarbonate buffer containing either 3.5 mM, 30 mM, or 50 mM KCl in the presence and absence of various adenosine analogs. Perfusates, collected every 2 min, were assayed for substance P by radioimmunoassay. Exposure of tissue to 50 mM KC1 produced an approximate three-fold increase in the release of substance P over basal release. This increase in release was calcium dependent. Perfusion of spinal cord tissues with either adenosine (10−3 M), N6-cyclohexyladenosine (10−5 M or 5 × 10−5 M), 5′-N-ethylcarboxamide adenosine (10−5 M) or L-N6-phenylisopropyladenosine (10−5 M) did not significantly alter basal or potassium-stimulated release of SP when compared to controls. In contrast to the adenosine agonists, exposure of the spinal cord tissue to 10−5 M morphine significantly reduced the potassium-stimulated release of substance P. Pretreatment of the slices with 10−5 M theophylline or 8-phenyltheophylline did not significantly attenuate the inhibition of substance P release produced by morphine. Theophylline alone (10−5 M) had no significant effect on either basal or potassium-stimulated release of SP. These studies demonstrate that adenosine does not inhibit the release of SP from spinal cord slices and does not appear to mediate the morphine-induced inhibition of SP release. The results suggest that the mechanism of the antinociceptive effects of adenosine at the level of the spinal cord is not via inhibition of substance P release.
Similar content being viewed by others
References
Braas KM, Newby AC, Wilson VS, Snyder SH (1986) Adenosine-containing neurons in the brain localized by immunocytochemistry. J Neurosci 6:1952–1961
Bruns RF, Lu GH, Pugsley TA (1986) Characterization of the A2 adenosine receptor labeled by [3H] NECA in rat striatal membranes. Mol Pharmacol 29:331–346
Chang HM, Berde CB, Holz GG, Steward GF, Kream RM (1989) Sufentanil, morphine, met-enkephalin, and k-agonist (U-50, 488H) inhibit substance P release from primary sensory neurons: A model for presynaptic spinal opioid actions. Anesthesiology 70:672–677
Choca JI, Proudfit HK, Green RD (1987) Identification of A1 and A2 adenosine receptors in the rat spinal cord. J Pharmacol Exp Ther 242:905–910
Choca JI, Green RD, Proudfit HK (1988) Adenosine A1 and A2 receptors of the substantia gelatinosa are located predominantly on intrinsic neurons: An autoradiography study. J Pharmacol Exp Ther 247:757–764
Cuello A (1987) Peptides as neuromodulators in primary sensory neurons. Neuropharmacology 26:971–979
Daly JW (1982) Adenosine receptors: targets for future drugs. J Med Chem 25:197–207
DeLander GE, Hopkins CJ (1986) Spinal adenosine modulates descending antinociceptive pathways stimulated by morphine. J Pharmacol Exp Ther 239:88–93
DeLander GE, Hopkins CJ (1987) Involvement of A2 adenosine receptors in spinal mechanisms of antinociception. Eur J Pharmacol 139:215–223
Dolphin AC, Forda SR, Scott RH (1986) Calcium-dependent currents in cultured rat dorsal root ganglion neurones are inhibited by an adenosine analogue. J Physiol (Lond) 373:47–61
Duggan AW, Hall JG, Headley PM (1977) Suppression of transmission of nociceptive impulses by morphine: selective effects of morphine administered into the region of the substantia gelatinosa. Br J Pharmacol 61:65–76
Einspahr FJ, Piercey MF (1980) Morphine depresses dorsal horn neuron responses to controlled noxious and non-noxious cutaneous stimulation. J Pharmacol Exp Ther 213:456–461
Fredholm BB, Dunwiddie TV (1988) How does adenosine inhibit transmitter release? Trends Pharmacol Sci 9:130–134
Gamse R, Leeman SE, Holzer P, Lembeck F (1981) Differential effects of capsaicin on the content of somatostatin, substance P and neurotensin in the nervous system of the rat. Naunyn-Schmiedeberg's Arch Pharmacol 317:140–148
Geiger JD, Nagy JI (1986) Distribution of adenosine deaminase activity in rat brain and spinal cord. J Neurosci 6:2707–2714
Geiger JD, LaBella FS, Nagy JI (1984) Characterization and localization of adenosine receptors in rat spinal cord. J Neuroscience 4:2303–2310
Go VCW, Yaksh TL (1987) Release of substance P from the cat spinal cord. J Physiol 391:141–167
Goodman RR, Snyder SH (1982) Autoradiographic localization of adenosine receptors in the rat brain using [3H]cyclohexyl-adenosine. J Neurosci 2:1230–1241
Herrick-Davis K, Chippari S, Luttinger D, Ward SJ (1989) Evaluation of adenosine agonists as potential analgesics. Eur J Pharmacol 162:365–369
Hökfelt T, Elde R, Johansson O, Luft R, Nilsson G, Arimura A (1976) Immunchistochemical evidence for separate populations of somatostatin-containing and substance P containing primary afferent neurons in the rat. Neuroscience 1:131–136
Hökfelt T, Kellerth JO, Nilsson G, Pernow B (1975) Substance P: localization in the central nervous system and in some primary sensory neurons. Science 190:889–890
Holmgren M, Hedner J, Mellstrand T, Nordberg G, Hedner Th (1986) Characterization of the antinociceptive effects of some adenosine analogues in the rat. Naunyn-Schmiedeberg's Arch Pharmacol 334:290–293
Holtz GG, Kream RM, Spiegel A, Dunlap K (1989) G proteins couple alpha-adrenergic and GABAB receptors to inhibition of peptide secretion from peripheral sensory neurons. J Neurosci 9:657–666
Illes P (1986) Mechanisms of receptor-mediated modulation of transmitter release in noradrenergic, cholinergic, and sensory neurones. Neuroscience 17:909–928
Jessell TM, Iversen LL (1977) Opiate analgesics inhibit substance P release from rat trigeminal nucleus. Nature 268:549–551
Jurna I (1981) Aminophylline differentiates between the depressant effects of morphine on spinal nociceptive reflex and on the spinal ascending activity from afferent C fibers. Eur J Pharmacol 71:393–400
Jurna I (1984) Cyclic nucleotides and aminophylline produce different effects on nociceptive motor and sensory responses in the rat spinal cord. Naunyn-Schmiedeberg's Arch Pharmacol 327:23–30
Kanazawa I, Sutoo D, Oshima I, Saito S (1979) Effect of transection on choline acetyltransferase, thyrotropin releasing hormone, and substance P in the cat cervical spinal cord. Neurosci Lett 13:325–330
Kuraishi Y, Hirota N, Sugimoto M, Satoh M, Takagi H (1983) Effect of morphine on noxious stimuli-induced release of substance P from rabbit dorsal horn in vivo. Life Sci 33:693–696
MacDonald RL, Skerritt JH, Werz MA (1986) Adenosine agonists reduce voltage-dependent calcium conductance of mouse sensory neurones in cell culture. J Physiol 370:75–90
Mauborgne A, Lutz O, Ledrand J-C, Hamon M, Cesselin F (1987) Opposite effects of delta and mu opioid receptor agonists on the in vitro release of substance P-like material from the rat spinal cord. J Neurochem 48:529–537
Mudge AW, Leeman SE, Fischbach GD (1979) Enkephalin inhibits release of substance P from sensory neurons in culture and decreases action potential duration. Proc Natl Acad Sci USA 76:526–530
Pang IH, Vasko MR (1986) Morphine and norepinephrine but not 5-hydroxytryptamine and gamma-aminobutyric acid inhibit the potassium-stimulated release of substance P from rat spinal cord slices. Brain Res 376:268–279
Post C (1984) Antinociceptive effects in mice after intrathecal injection of 5′-N-ethylcarboxamide adenosine. Neurosci Lett 51:325–330
Rodbard RJ, Lewald JE (1970) Computer analysis of radioligand assay and radioimmunoassay data. Acta Endocrinol Suppl 146, 64:79–103
Salt TE, Hill RG (1983) Neurotransmitter candidates of somatosensory primary afferent fibres. Neurosci 4:1083–1103
Sawynok J, Sweeney MI, White TD (1986) Classification of adenosine receptors mediating antinocieeption in the rat spinal cord. Br J Pharmacol 88:923–930
Sawynok J, Sweeney MI, White TD (1989) Adenosine release may mediate spinal analgesia by morphine. Trends Pharmacol Sci 10:186–189
Sweeney MI, White TD, Sawynok J (1987) Involvement of adenosine in the spinal antinociceptive effects of morphine and noradrenaline. J Pharmacol Exp Ther 243:657–665
Sweeney MI, White TD, Sawynok J (1989) Morphine, capsaicin and K+ release purines from capsaicin-sensitive primary afferent nerve terminals in the spinal cord. J Pharmacol Exp Ther 248:447–454
Tessler A, Himes BT, Soper K, Murray M, Goldberger ME, Reichlin S (1984) Recovery of substance P but not somatostatin in the cat spinal cord after unilateral lumbosacral dorsal rhizotomy: A quantitative study. Brain Res 305:95–102
Author information
Authors and Affiliations
Additional information
Send offprint requests to M. R. Vasko at the above address
Rights and permissions
About this article
Cite this article
Vasko, M.R., Ono, H. Adenosine analogs do not inhibit the potassium-stimulated release of substance P from rat spinal cord slices. Naunyn-Schmiedeberg's Arch Pharmacol 342, 441–446 (1990). https://doi.org/10.1007/BF00169462
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00169462