Abstract
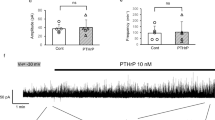
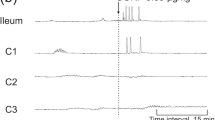
We aimed at studying the mechanism(s) of the inhibitory effect exerted by calcitonin gene-related peptide (CGRP) on the spontaneous activity of the guinea-pig isolated renal pelvis. In organ bath experiments, CGRP (1–100 nM) produced a concentration-dependent (EC50 8 nM) partial inhibition (Emax about 35% inhibition of motility index) of spontaneous contractions. The potassium (K) channel opener, cromakalim (3–10 μM) promptly suppressed the spontaneous contractions in a glibenclamide- (10 μM) sensitive manner. Glibenclamide (10 μM) did not affect the inhibitory action of CGRP. The calcium (Ca) channel agonist, Bay K 8644 (1 μM), markedly enhanced the spontaneous activity of the renal pelvis and reduced the inhibitory effect of CGRP. The protein kinase A inhibitors Rp-cAMPS (300 μM), H8 (100 μM) and H89 (10 μM), and the blockers of intracellular Ca handling bysarcoplasmic reticulum, ryanodine (100 μM) and thapsigargin (1 μM) did not affect the response to CGRP. The response to CGRP was likewise unaffected by the nitric oxide synthase inhibitor, L-nitroarginine (30 μM) and by the protein kinase G inhibitor, KT5823 (3 μM). Furthermore, the inhibitory action of CGRP was not modified by lowering the extracellular concentration of K (from 5.9 to 1.2 mM) nor by increasing (from 2.5 to 3.75 mM) or decreasing (from 2.5 to 0.25 mM) the extracellular Ca concentration. Replacement of 80% glucose with 2-deoxyglucose (2-DOG) reduced the amplitude of spontaneous contractions, both in the absence and presence of 10 μM glibenclamide. In the presence of 2-DOG, the inhibitory action of CGRP was enhanced at a similar extent, either in the absence or presence of glibenclamide. In sucrose gap, the effect of CGRP (0.1 μM for 5 min) was separately analyzed in the proximal (close to the kidney) and distal (close to the ureter) regions of the renal pelvis. Both preparations discharged spontaneous (pacemaker) action potentials having different shape, duration and frequency. CGRP had no effect on pacemaker potentials in the proximal renal pelvis while producing about 30% reduction of the frequency of pacemaker potentials and motility index in the distal renal pelvis. Cromakalim (3 μM) abolished pacemaker potentials in both regions of the renal pelvis.
In conjunction with the results of previous studies in the guinea-pig ureter, the present findings document the existence of remarkable regional differences in the effector mechanisms initiated by CGRP receptor occupancy in the guinea-pig pyeloureteral tract. CGRP appears to be inherently unable to activate glibenclamide-sensitive K channels in the guinea-pig renal pelvis, a mechanism which is central for its ability to suppress latent pacemakers in the ureter. Within the renal pelvis, the sensitivity to the inhibitory effect of CGRP appears in the more distal region, from which an ‘ureter-like’ action potential is recorded.
Similar content being viewed by others
References
Artemenko DP, Bury VA, Vladimirova IA, Shuba MF (1982) Modification of the single sucrose-gap method Physiol Zhurn 28:374–380
Ashcroft SJH, Ashcroft FM (1990) Properties and functions of ATP-sensitive K channels. Cell Signalling 2:197–214
Bonev AD, Nelson MT (1993) ATP-sensitive potassium channels in smooth muscle cells from guinea-pig urinary bladder. Am J Physiol 264:C1190-C1200
Constantinou CE, Neubarth JL, Mensah-Dwumah M (1977) Frequency gradient in the autorhyhtmicity of the pyeloureteral pacemaker system. Experientia 34:614–615
Gosling JA, Dixon JS (1972) Structural evidencxe in support of an urinary tract pacemaker. Br J Urol 44:550–560
Gosling JA, Dixon JS (1974) Species variations in the location of upper urinary tract pacemaker cells. Invest Urol 11:418–423
Hidaka H, Kobayashi R (1992) Pharmacology of protein kinase inhibitors. Annu Rev Pharmacol Toxicol 32:377–397
Hoyle CHV (1987) A modified single sucrose gap -junction potentials and electrotonic potentials in gastrointestinal smooth muscle. J Pharmacol Methods 18:219–226
Hua XY, Saria A, Gamse R, Theodorsson-Norheim E, Brodin E, Lundberg JM (1986) Capsaicin-induced release of multiple tachykinins (SP, neurokinin A and eledoisin-like material) from guinea-pig spinal cord and ureter. Neuroscience 19:313–319
Hua XY, Theodorsson-Norheim E, Lundberg JM, Kinn AC, Hokfelt T, Cuello AC (1987) Co-localization of tachykinins and CGRP in capsaicin-sensitive afferents in relation to motility effects in the human ureter in vitro. Neuroscience 23:693–703
Jiang H, Colbran JL, Francis SH, Corbin JD (1992) Direct evidence for cross-linking of cGMP-dependent protein kinase by cAMP in pig coronary arteries. J Biol Chem 267:1015–1019
Kimoto Y, Constantinou CE (1991) Regional effects of indomethacin, acetylsalycilic acid and SC19220 on the contractility of rabbit renal pelvis (pacemaker regions and pelviureteric junction). J Urol 146:433–438
Lincoln TM, Cornwell TL, Taylor AE (1990) cGMP-dependent protein kinase mediates the reduction of calcium by cAMP in vascular smooth muscle cells. Am J Physiol 258:C399-C407
Maggi CA (1995) Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog Neurobiol 45:1–98
Maggi CA, Giuliani S (1991) The neurotransmitter role of CGRP in the rat and guinea-pig ureter: effect of a CGRP antagonist and species-related differences in the action of omega conotoxin on CGRP release from primary afferents. Neuroscience 43:261–271
Maggi CA, Giuliani S (1992) NANC excitatory innervation of the guinea-pig isolated renal pelvis: involvement of capsaicin-sensitive primary afferent neurons. J Urol 147:1394–1398
Maggi CA, Giuliani S (1994a) A thiorphan-sensitive mechanism regulates the action of both exogenous and endogenous CGRP in the guinea-pig ureter. Regul Pept 51:263–271
Maggi CA, Giuliani S (1994b) CGRP regulates excitability and refractory period of the guinea-pig ureter. J Urol 152:520–524
Maggi CA, Meli A (1988) The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol 19:1–13
Maggi CA, Santicioli P, Patacchini R, Geppetti P, Giuliani S, Astolfi M, Baldi E, Parlani M, Theodorsson E, Fusco B, Meli A (1988) Regional differences in the motor response to capsaicin in the guinea-pig urinary bladder: relative role of pre- and postjunctional factors related to neuropeptide-containing sensory nerves. Neuroscience 27:675–688
Maggi CA, Tramontana M, Cecconi R, Santicioli P (1990) Neurochemical evidence for the involvement of N-type calcium channels in transmitter secretion from peripheral endings of sensory nerves in guinea-pigs. Neurosci Lett 114:203–206
Maggi CA, Giuliani S, Del Bianco E, Geppetti P, Theodorsson E, Santicioli P (1992a) CGRP in the regulation of urinary tract motility. Ann NY Acad Sci 657:328–343
Maggi CA, Santicioli P, Del Bianco EeE, Giuliani S (1992b) Local motor responses to bradykinin and bacterial chemotactic peptide FMLP in the guinea-pig isolated renal pelvis and ureter. J Urol 148:1944–1950
Maggi CA, Theodorsson E, Santicioli P, Giuliani S (1992c) Tachykinins and CGRP as co-transmitters in local motor responses produced by sensory nerve activation in the guinea-pig isolated renal pelvis. Neuroscience 46:549–559
Maggi CA, Giuliani S, Santicioli P (1994a) Multiple mechanisms in the smooth muscle relaxant action of calcitonin gene-related peptide (CGRP) in the guinea-pig ureter. Naunyn Schmiedeberg's Arch Pharmacol 350:537–547
Maggi CA, Giuliani S, Santicioli P (1994b) Effect of cromakalim and glibenclamide on spontaneous and evoked motility of the guinea-pig isolated renal pelvis and ureter. Br J Pharmacol 111:687–694
Maggi CA, Giuliani S, Santicioli P (1994c) Effect of Bay K 8644 and ryanodine on the refractory period, action potential and mechanical response of the guinea-pig ureter to electrical stimulation. Naunyn Schmiedeberg's Arch Pharmacol 349:510–522
Maggi CA, Giuliani S, Santicioli P (1995) Effect of the Ca2+-ATPase inhibitor, cyclopiazonic acid, on electromechanical coupling in the guinea-pig ureter. Br J Pharmacol 114:127–137
Maggi CA, Patacchini R, Eglezos A, Quartara L, Giuliani S, Giachetti A (1992d) Tachykinin receptors in guinea-pig renal pelvis: activation by exogenous and endogenous tachykinins. Br J Pharmacol 107:27–33
Meini S, Santicioli P, Maggi CA (1995) Propagation of impulses in the guinea-pig ureter and its blockade by calcitonin gene-related peptide (CGRP). Naunyn Schmiedeberg's Arch Pharmacol 351:79–86
Murray KJ (1990) cAMP and mechanisms of vasodilatation. Pharmac Ther 47:329–345
Nichols CG, Lederer WJ (1991) ATP-sensitive potassium channels in the cardiovascular system. Am J Physiol 261:H1675-H1686
Nishimura J, Van Breemen C (1989) Direct regulation of smooth muscle contractile elements by second messengers. Biochem Biophys Res Commun 163:929–935
Santicioli P, Maggi CA (1994) Inhibitory transmitter action of CGRP in the guinea-pig ureter via activation of glibenclamide-sensitive K+ channels. Br J Pharmacol 113:588–592
Santicioli P, Morbidelli L, Parenti A, Ziche M, Maggi CA (1995a) CGRP selectively increases cAMP levels in the guinea-pig ureter. Eur J Pharmacol - Mol Pharmacol 289:17–21
Santicioli P, Carganico G, Meini S, Giuliani S, Giachetti A, Maggi CA (1995b) Modulation by stereoselective inhibition of cyclooxygenase of electromechanical coupling in the guinea-pig isolated renal pelvis. Br J Pharmacol 114:1149–1158
Su HC, Wharton J, Polak JM, Mulderry PK, Ghatei MA, Gibson SJ, Terenghi G, Morrison JFB, Ballesta J, Bloom SR (1986) CGRP immunoreactivity in afferent neurons supplying the urinary tract: combined retrograde tracing and immunohistochemistry. Neuroscience 18:727–747
Tamaki M, Iwanaga T, Sato S, Fujita T (1992) CGRP immunoreactive nerve plexuses in the renal pelvis and ureter of rats. Cell Tissue Res 267:29–33
Van Hasstert PJM, Van Driel R, Jastorff B, Baraniak J, Steck WJ, De Wit RJW (1984) Competitive cAMP antagonists for cAMP receptor proteins. J Biol Chem 259:10020–10024
Zawalinski VC, Constantinou CE, Burnstock G (1975) Ureteral pacemaker potentials recorded with the sucrose gap technique. Experientia 531:931–933
Zhang Y, Lang RJ (1994) Effects of intrinsic prostaglandins on the spontaneous contractile and electrical activity of the proximal renal pelvis of the guinea-pig. Br J Pharmacol 113:431–439
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maggi, C.A., Giuliani, S. & Santicioli, P. CGRP inhibition of electromechanical coupling in the guinea-pig isolated renal pelvis. Naunyn-Schmiedeberg's Arch Pharmacol 352, 529–537 (1995). https://doi.org/10.1007/BF00169387
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00169387