Summary
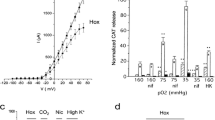
To clarify the effects of hypoxia on stimulus-release coupling, we have examined the effects of hypoxia on nicotine-induced catecholamine (noradrenaline and adrenaline) release from, and 22Na+ influx, 45Ca2+ influx and cytosolic free Ca2+ concentration ([Ca2+]i) in, cultured bovine adrenal chromaffin cells. Experiments were carried out in media pre-equilibrated with 21% O2/79% N2 (control) or with 0% O2/100% N2 (hypoxia). Cells were stimulated with either nicotine (activating nicotinic acetylcholine (ACh) receptors) or a high K+ concentration (55 mmol/1 KCI; directly activating voltage-dependent Ca2+ channels). Hypoxia reduced both nicotine- and high K+-induced catecholamine releases from the cells, but the reduction of the former (to about 30% of the control value) was more pronounced than that of the latter (to about 40% of the control value). Nicotine-induced 22Na+ influx, which is considered to reflect the function of nicotinic ACh receptors, was inhibited by hypoxia. Both nicotine- and high K+-induced 45Ca2+ influx into the cells were reduced by hypoxia, but the reduction of the former was more pronounced than that of the latter. Nicotine- and high K+-induced increases in [Ca2+]i were reduced by hypoxia to about 30% and 40% of the control values, respectively. These results suggest that hypoxia reduces cation influxes (Na+ and Ca2+) through both the ligand-gated cation channels of the nicotinic ACh receptor and the voltage-dependent Ca2+ channels.
Similar content being viewed by others
References
Amy C, Kirshner N (1982) 22Na+ uptake and catecholamine secretion by primary cultures of adrenal medulla cells. J Neurochem 39:132–142
Artalejo CR, Garcia AG, Aunis D (1987) Chromaffin cell calcium channel kinetics measured isotopically through fast calcium, strontium, and barium fluxes. J Biol Chem 262:915–926
Baker PF, Rink TJ (1975) Catecholamine release from bovine adrenal medulla in response to maintained depolarization. J Physiol (Lond) 253:593–620
Boksa P and Livett BG (1984) Desensitization to nicotinic cholinergic agonists and K+, agents that stimulate catecholamine secretion, in isolated adrenal chromaffin cells. J Neurochem 42:607–617
Borle AB (1981) Pitfalls of the 45Ca uptake method. Cell Calcium 2:187–196
Catterall WA, Nirenberg M (1973) Sodium uptake associated with activation of action potential ionophores of cultured neuroblastoma and muscle cells. Proc Natl Acad Sci USA 70:3759–3763
Couraud F, Martin-Moutot N, Koulakoff A, Berwald-Netter Y (1986) Neurotoxin-sensitive sodium channels in neurons developing in vivo and in vitro. J Neurosci 6:192–198
Daly JW, Nishizawa Y, Edwards MW, Waters JA, Aronstam RS (1991) Nicotinic receptor-elicited sodium flux in rat pheochromocytoma PC12 cells: effects of agonists, antagonists, and noncompetitive blockers. Neurochem Res 16:489–500
Decker ER, Dani JA (1990) Calcium permeability of the nicotinic acetylcholine receptor: the single-channel calcium influx is significant. J Neurosci 10:3413–3420
Douglas WW (1968) Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol 34:451–474
Douglas WW Kanno T, Sampson SR (1967) Influence of the ionic environment on the membrane potential of adrenal chromaffin cells and on the depolarizing effect of acetylcholine. J Physiol (Lond) 191:107–121
Downing JEG, Role LW (1987) Activators of protein kinase C enhance acetylcholine receptor desensitization in sympathetic ganglion neurons. Proc Natl Acad Sci USA 84:7739–7743
Fisher SK, Holz RW, Agranoff BW (1981) Muscarinic receptors in chromaffin cell cultures mediate channel phospholipid labeling but not catecholamine secretion. J Neurochem 37:491–497
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Higgins LS, Berg DK (1988a) A desensitized form of neuronal acetylcholine receptor detected by 3H-nicotine binding on bovine adrenal chromaffin cells. J Neurosci 8:1436–1446
Higgins LS, Berg DK (1988b) Cyclic AMP-dependent mechanism regulates acetylcholine receptor function on bovine adrenal chromaffin cells and discriminates between new and old receptors. J Cell Biol 107:1157–1165
Holz RW, Senter RA, Frye RA (1982) Relationship between Ca2+ uptake and catecholamine secretion in primary dissociated cultures of adrenal medulla. J Neurochem 39:635–646
Kao L-S, Schneider AS (1986) Calcium mobilization and catecholamine secretion in adrenal chromaffin cells: a quin-2 fluorescence study. J Biol Chem 261:4881–4888
Kidokoro Y, Ritchie AK (1980) Chromaffin cell action potentials and their possible role in adrenaline secretion from the rat adrenal medulla. J Physiol (Lond) 307:199–266
Kilpatrick DL, Slepetis RJ, Corcoran JJ, Kirshner N (1982) Calcium uptake and catecholamine secretion by cultured bovine adrenal medulla cells. J Neurochem 38:427–435
Knight DE, Kesteven NT (1983) Evoked transient intracellular free Ca2+ changes and secretion in isolated bovine adrenal medullary cells. Proc R Soc Lond [Biol] 218:177–199
Lee K, Miwa S, Fujiwara M, Magaribuchi T, Fujiwara M (1987) Differential effects of hypoxia on the turnover of norepinephrine and epinephrine in the heart, adrenal gland, submaxillary gland and stomach. J Pharmacol Exp Ther 240:954–958
Lee K, Miwa S, Hayashi Y, Koshimura K, Taniguchi T, Orii Y, Fujiwara M (1988) Effects of hypoxia on contractile responses of rabbit aortic strips to transmural electrical stimulation. Naunyn-Schmiedebergs Arch Pharmacol 338:275–281
Lee K, Miwa S, Hayashi Y, Koshimura K, Fujiwara M, Orii Y (1989) Effects of hypoxia on noradrenaline release and neuronal reuptake in isolated rabbit thoracic aortic strips. Naunyn-Schmiedebergs Arch Pharmacol 339:503–508
Lee K, Miwa S, Koshimura K, Hasegawa H, Hamahata K, Fujiwara M (1990) Effects of hypoxia on the catecholamine release, Ca2+ uptake and cytosolic free Ca2+ concentration in cultured bovine adrenal chromaffin cells. J Neurochem 55:1131–1137
Lee K, Miwa S, Koshimura K, Ito A (1992) Characterization of nicotinic acetylcholine receptors on cultured bovine adrenal chromaffin cells using modified L-[3H]nicotine binding assay. Naunyn-Schmiedebergs Arch Pharmacol 345:363–369
Miwa S, Fujiwara M, Inoue M, Fujiwara M (1986) Effects of hypoxia on the activities of noradrenergic and dopaminergic neurons in the rat brain. J Neurochem 47:63–69
Role LW Leeman SE, Perlman RL (1981) Somatostatin and substance P inhibit catecholamine secretion from isolated cells of guinea-pig adrenal medulla. Neuroscience 6:1813–1821
Rudy B, Kirschenbaum B, Rukenstein A, Greene LA (1987) Nerve growth factor increases the number of functional Na channels and induces TTX-resistant Na channels in PC12 pheochromocytoma cells. J Neurosci 7:1613–1625
Sands SB, Barish ME (1991) Calcium permeability of neuronal nicotinic acetylcholine receptor channels in PC12 cells. Brain Res 560:38–42
Simmons LK, Schuetze SM, Role LW (1990) Substance P modulates single-channel properties of neuronal nicotinic acetylcholine receptors. Neuron 2:393–403
Stallcup WB (1979) Sodium. and calcium fluxes in a clonal nerve cell line. J Physiol (Lond) 286:525–540
Stallcup WB, Patrick J (1980) Substance P enhances cholinergic receptor desensitization in a clonal nerve cell line. Proc Natl Acad Sci USA 77:634–638
Vernino S, Amador M, Luetje CW, Patrick J, Dani JA (1992) Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron 8:127–134
Wada A, Takara H, Izumi F, Kobayashi H, Yanagihara N (1985) Influx of 22Na through acetylcholine receptor-associated Na channels: relationship between 22Na influx, 45Ca influx and secretion of catecholamines in cultured bovine adrenal medulla cells. Neuroscience 15:283–292
Wada A, Takara H, Yanagihara N, Kobayashi H, Izumi F (1986) Inhibition of Na+-pump enhances carbachol-induced influx of 45Ca2+ and secretion of catecholamines by elevation of cellular accumulation of 22Na+ in cultured bovine adrenal medullary cells. Naunyn-Schmiedebergs Arch Pharmacol 332:351–356
White TD, Bourke JE, Livett BG (1987) Direct and continuous detection of ATP secretion from primary monolayer cultures of bovine adrenal chromaffin cells. J Neurochem 49:1266–1273
Author information
Authors and Affiliations
Additional information
Correspondence to K. Lee at the present address
Rights and permissions
About this article
Cite this article
Lee, K., Sekine, A. Effects of hypoxia on stimulus-release coupling mechanisms in cultured bovine adrenal chromaffin cells. Naunyn-Schmiedeberg's Arch Pharmacol 348, 275–281 (1993). https://doi.org/10.1007/BF00169156
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00169156