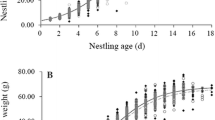
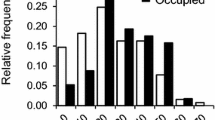
Abstract
Antechinuses (Dasyuridae: Marsupialia) exhibit dramatic interpopulation variation in the sex ratio at birth, a pattern which has previously been interpreted in terms of both local resource competition and Trivers/Willard effects. However, Antechinus stuartii usually fail to wean all the young that attach to their teats. At least in captivity, this is because they often eat their young. In free-living populations, brood reduction affects sons and daughters differently. Mothers virtually always wean some daughters. The probability that a daughter will be weaned declines with the number of daughters in the pouch. The health or quality of the mother does not affect the number of daughters weaned. By contrast, mothers tend to wean all or none of their sons. A strong correlate of infanticide against sons is senescence. Old mothers rarely invest in sons, and produce low-quality daughters. Mothers suffer a direct cost (mortality during lace lactation) of male-biased litters. Coupled with data on prenatal sex allocation, these results support the conjoint influence of local resource competition and the Trivers-Willard effect. However, they suggest that in populations where females are largely semelparous, the population optimum generated by local resource competition may be unattainable, because of the importance of producing at least one daughter. These observations support recent theoretical claims that the sex ratio at the population level is not easily predicted, but suggest that the diversity of mammalian sex allocation tactics has been underestimated.
Similar content being viewed by others
References
Aitkin M, Anderson D, Francis B, Hinde J (1989) Statistical modelling in GLIM. Clarendon, Oxford
Albon SD, Clutton-Brock TH (1988) Climate and the population dynamics of red deer in Scotland. In: Usher MB, Thompson DBA (eds) Ecological change in the uplands. Blackwell Scientific, Oxford, pp 93–107
Albon SD, Clutton-Brock TH, Guinness FE (1987) Early development and population dynamics in red deer. 11. Density-independent effects and cohort variation. J Anim Ecol 56:69–81
Altmann J (1980) Baboon mothers and infants. Harvard University Press, Cambridge
Armitage KB (1987) Do female yellow-bellied marmots adjust the sex ratios of their offspring? Am Nat 129:501–519
Austad SN, Sundquist ME (1986) Sex-ratio manipulation in the common opossum. Science 324:58–60
Bryant DM (1989) Determination of respiration rates of free-living animals by the doubly-labelled technique. In: Grubb PJ, Whittaker JB (eds) Towards a more exact ecology Blackwell Scientific, Oxford, pp 85–109
Bulmer MG (1986) Sex ratio theory in geographically structured populations. Heredity 56:69–73
Charnov EL (1982) The theory of sex allocation. Princeton University Press, Princeton
Charnov EL, Schaffer WM (1973) Life history consequences of natural selection: Cole's result revisited. Am Nat 107:291–303
Clark AB (1978) Sex ratio and local resource competition in a prosimian primate. Science 201:163–165
Clark MM, Spencer CA, Galef BG Jr (1986) Reproductive life history correlates of early and late sexual maturation in female Mongolian gerbils (Meriones unguiculatus). Anim Behav 34:551–560
Clark MM, Bone S, Galef BG (1990) Evidence of sex-biased postnatal investment by Mongolian gerbils. Anim Behav 39:735–744
Clark MM, Waddingham CL, Galef BG (1991) Further evidence of sex-biased maternal investment by Mongolian gerbil dams. Anim Behav 42:161–162
Clutton-Brock TH (1988) Reproductive success. In: Clutton-Brock TH (ed) Reproductive success: studies on individual variation in contrasting breeding systems. University of Chicago Press, Chicago, pp 472–485
Clutton-Brock TH (1991) The evolution of parental care. Princeton University Press, Princeton
Clutton-Brock TH, Iason GR (1986) Sex ratio variation in mammals. Q Rev Biol 61:339–374
Clutton-Brock TH, Albon SD, Guinness FE (1981) Parental investment in male and female offspring in polygynous mammals. Nature 289:487–489
Clutton-Brock TH, Albon SD, Guinness FE (1985) Parental investment and sex differences in juvenile mortality in birds and mammals. Nature 313:131–133
Clutton-Brock TH, Albon SD, Guinness FE (1986) Great expectations: maternal dominance, sex ratios and offspring reproductive success in red deer. Anim Behav 34:460–471
Cockburn A (1989) Adaptive patterns in marsupial reproduction. Trends Ecol Evol 4:126–131
Cockburn A (1990) Sex ratio variation in marsupials. Aust J Zool 37:467–479
Cockburn A (1992) The duration of lactation in Antechinus stuartii (Marsupialia, Dasyuridae). Aust J Zool 40:195–204
Cockburn A, Lazenby-Cohen KA (1992) Use of nest trees by Antechinus stuartii, a semelparous lekking marsupial. J Zool London 226:657–680
Cockburn A, Lee AK, Martin RW (1983) Macrogeographic variation in litter size in Antechinus (Marsupialia: Dasyuridae). Evolution 37:86–95
Cockburn A, Scott MP, Dickman CR (1985a) Sex ratio and intra-sexual kin competition in mammals. Oecologia 66:427–429
Cockburn A, Scott MP, Scotts DJ (1985b) Inbreeding avoidance and male-biased natal dispersal in Antechinus spp. (Marsupialia: Dasyuridae). Anim Behav 33:908–915
Cole LC (1954) The population consequences of life history phenomena. Q Rev Biol 29:103–137
Comins HN, Hamilton WD, May RM (1980) Evolutionarily stable dispersal strategies. J Theor Biol 82:205–230
Crowley HM (1990) Energetics of lactation in two species of Antechinus. PhD thesis, Australian National University
Dickman CR (1986) An experimental study of competition between two species of dasyurid marsupials. Ecol Monogr 56:221–241
Dickman CR (1988) Sex-ratio variation in response to inter-specific competition. Am Nat 132:289–297
Drent RH, Daan S (1980) The prudent parent: energetic adjustments in avian breeding. Ardea 68:225–252
Fairbanks LA, McGuire MT (1986) Age, reproductive value and dominance related behaviour in vervet monkey females: cross-generational social influences on social relationships and reproduction. Anim Behav 34:1718–1721
Frank SA (1987) Individual and population sex allocation patterns. Theor Popul Biol 31:47–74
Frank SA (1990) Sex allocation theory for birds and mammals. Annu Rev Ecol Syst 21:13–55
Genstat 5 Committee (1987) Genstat 5 Reference Manual. Clarendon, Oxford
Gomendio M, Clutton-Brock TH, Albon SD, Guinness FE, Simpson MJ (1990) Mammalian sex ratios and variation in costs of rearing sons and daughters. Nature 343:261–263
Greenwood PJ (1980) Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 28:1140–1162
Haig D (1990) Brood reduction and optimal parental investment when offspring differ in quality. Am Nat 136:550–566
Hogg JT, Hass CC, Jenni DA (1992) Sex-biased maternal expenditure in Rocky Mountain bighorn sheep. Behav Ecol Sociobiol 31:243–251
Johnson CN (1988) Dispersal and the sex ratio at birth in primates. Nature 332:726–728
Kasuya T, Marsh H (1984) Life history and reproductive biology of the short-finned pilot whale, Globicephala macrohynchus, off the Pacific coast of Japan. Rep Int Whal Comm Special 6:259–310
Kojola I, Eloranta E (1989) Influences of maternal body weight, age, and parity on sex ratio in semidomesticated reindeer (Rangifer t. tarandus). Evolution 43:1331–1336
Labov JB, Huck VW, Vaswani P, Lisk RD (1986) Sex ratio manipulation and decreased growth of male offspring of un dernourished golden hamsters (Mesocricetus auratus). Behav Ecol Socbiol 18:241–249
Lazenby-Cohen KA (1991) Communal nesting in Antechinus stuartii (Marsupialia: Dasyuridae). Aust J Zool 39:273–283
Lazenby-Cohen KA, Cockburn A (1988) Lek promiscuity in a semelparous mammal, Antechinus stuartii (Marsupialia: Dasyuridae)? Behav Ecol Sociobiol 22:195–202
Lazenby-Cohen KA, Cockburn A (1991) Social and foraging components of the home range in Antechinus stuartii (Dasyuridae: Marsupialia). Aust J Ecol 16:301–307
Le Boeuf BJ, Condit R, Reiter J (1989) Parental investment and the secondary sex ratio in northern elephant seals. Behav Ecol Sociobiol 25:109–117
Lee AK, Cockburn A (1985) Evolutionary ecology of marsupials. Cambridge University Press, Cambridge
Low BS (1978) Environmental uncertainty and the parental strategies of marsupials and eutherians. Am Nat 112:197–213
Marsh H, Kasuya T (1984) Changes in the ovaries of the short-finned pilot whale, Globicephala macrorhynchus, with age and reproductive activity. Rep Int Whale Comm Special 6:311–335
McLure PA (1981) Sex-biased litter reduction in food-restricted wood rats (Neotoma floridana). Science 211:1058–1060
McShea WJ, Madison DM (1986) Sex ratio shifts within litters of meadow voles (Microtus pennsylvanicus). Behav Ecol Sociobiol 18:431–436
Newton I (1989) Synthesis. In: Newton I (ed) Lifetime reproduction in birds. Academic Press, London, pp 441–469
Paul A, Kuester J (1990) Adaptive significance of sex ratio adjustment in semifree-ranging Barbary macaques (Macacus sylvanus) at Salem. Behav Ecol Sociobiol 27:287–293
Perret M (1990) Influence of social factors on sex ratio at birth, maternal investment and young survival in a prosimian primate. Behav Ecol Sociobiol 27:447–454
Peterson CC, Nagy KA, Diamond J (1990) Sustainable metabolic scope. Proc Natl Acad Sci USA 87:2324–2328
Schaik CP van, Hrdy SB (1991) Intensity of local resource competition shapes the relationship between maternal rank and sex ratios at birth in cercopthecine primates. Am Nat 138:1555–1562
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary the sex ratio of offspring. Science 179:90–92
Tyndale-Biscoe CH, Renfree MB (1987) Reproductive physiology of marsupials. Cambridge University Press, Cambridge
Wasser SK, Norton G (1993) Baboons adjust secondary sex ratio in response to predictors of sex-specific offspring survival. Behav Ecol Sociobiol 32:273–281
Williams GC (1979) The question of adaptive sex-ratio in out-crossed vertebrates. Prod R Soc London B 205:567–580
Young TP (1981) A general model of comparative fecundity for semelparous and iteroparous life histories. Am Nat 118:27–36
Author information
Authors and Affiliations
Additional information
Communicated by P. Harvey
Rights and permissions
About this article
Cite this article
Cockburn, A. Adaptive sex allocation by brood reduction in antechinuses. Behav Ecol Sociobiol 35, 53–62 (1994). https://doi.org/10.1007/BF00167060
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00167060