Summary
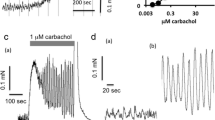
To analyse the potency of glycopyrronium bromide in blocking responses mediated via subtypes of muscarinic receptors in vitro, we tried to determine its equilibrium dissociation constants at prejunctional muscarinic receptors inhibiting the twitch response of rabbit vas deferens (presumed M1 type), at M2 (paced rat left atria), M3 (guinea pig ileum) muscarinic receptor subtypes and at the muscarinic receptor of the rabbit iris sphincter (not M1–M4, not m5). Glycopyrronium bromide shifted to the right the curve for inhibition of the twitch response induced by the agonist McN-A-343, and the methacholine-induced curves for inhibition of rat atrial contraction, and for tonic contraction of guinea pig ileum and rabbit iris sphincter.
Glycopyrronium bromide blocked with very high potency (> 11, apparent -log KB) the response in rabbit vas deferens. Its affinity was low (9.09) for the M2 subtype, and intermediate (10.31 or 10.13) for the ileal M3 and the atypical iris muscarinic receptor subtype, respectively. Except at the receptors in rabbit vas deferens, the blockade of agonist effect appeared to be of simple competitive type.
In conclusion, glycopyrronium bromide is about 10 or 100fold more potent in preventing a response to activation of the prejunctional receptor in rabbit vas deferens than in blocking an M3 or M2 muscarinic receptor subtype, respectively, in vitro. The low affinity for M2 receptors may, in part, explain the low incidence of unwanted tachycardia in therapy. The drug failed to discriminate between an M3 receptor and the atypical rabbit iris sphincter receptor.
Similar content being viewed by others
References
Ali-Melkkila T, Kaila T, Kanto J (1989) Glycopyrrolate: pharmacokinetics and some pharmacodynamic findings. Acta Anaesthesiol Scand 33:513–517
Ali-Melkkila T, Kaila T, Antila K, Halkola L, lisalo E (1991) Effects of glycopyrrolate and atropine on heart rate variability. Acta Anaesthesiol Scand 35:436–441
Arunlakshana O, Schild HO (1959) Some quantitative uses of drug antagonists. Br J Pharmacol 14:48–58
Barlow RB, Berry KJ, Glenton PAM, Nikolaou NM, Soh KS (1976) A comparison of affinity constants for muscarine sensitive acetylcholine receptors in guinea-pig atrial pacemaker cells at 29'C and in ileum at 29°C and 37°C. Br J Pharmacol 58:613–620
Berger J, Gravenstein J, van der Aa J, Paulus L, Sabah-Maren E, McLaughlin G (1988) Comparative potency of atropine sulphate and glycopyrrolate on heart rate in man. Eur J Anaesthesiol 5:23–30 Bognar IT, Altes U, Beinhauer C, Kessler I, Fuder H (1992) A muscarinic receptor different from M1, M2, M3 and M4 subtypes mediates the contraction of the rabbit iris sphincter. Naunyn-Schmiedeberg's Arch Pharmacol 345:611–618
Doods HN (1991) Muscarinic receptors. In: Doods HN, van Meel JCA (eds) Receptor data for biological experiments: a guide to drug selectivity. Ellis Howood, New York, pp 31–34
Dörje F, Wess J, Lambrecht G, Tacke R, Mutschler E, Brann MR (1991) Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J Pharmacol Exp Ther 256:727–733
Durant PAC, Shankley NP, Welsh NJ, Black JW (1991) Pharmacological analysis of antagonist interactions at acetylcholine muscarinic receptors in a new urinary bladder assay. Br J Pharmacol 104:145–150
Dworacek B, Rupreht J, Bode A, Walhout MJ (1987) Atropine methylbromide and glycopyrrolate: a comparative study during reversal of neuromuscular block. Acta Anaesthesiol Belg 38:147–152
Eglen RM, Whiting RL (1986) Muscarinic receptor subtypes: a critique of the current classification and a proposal for a working nomenclature. J Anton Pharmacol 6:323–346
Eglen RM, Whiting RL (1988) Comparison of the muscarinic receptors of the guinea-pig oesophageal muscularis mucosae and trachea in vitro. J Anton Pharmacol 8:181–189
Eltze M (1988) Muscarinic M1- and M2-receptors mediating opposite effects on neuromuscular transmission in rabbit vas deferens. Eur J Pharmacol 151:205–221
Eltze M, Figala V (1988) Affinity and selectivity of biperiden enantiomers for muscarinic receptor subtypes. Eur J Pharmacol 158:11–19
Fuder H, Kilbinger H, Müller H (1985) Organ selectivity of hexahydrosiladifenidol in blocking pre- and postjunctional muscarinic receptors studied in guinea-pig ileum and rat heart. Eur J Pharmacol 113:125–127
Fuder H, SchÖpf J, Unckell J, Wesner MTh, Melchiorre C, Tacke R, Mutschler E, Lambrecht G (1989) Different muscarine receptors mediate the prejunctional inhibition of [3H]-noradrenaline release in rat or guinea-pig iris and the contraction of the rabbit iris sphincter muscle. Naunyn-Schmiedeberg's Arch Pharmacol 340:597–604
Gilman MJ, Meyer L, Carter J, Slovis C (1990) Comparison of aerosolized glycopyrrolate and metaproterenol in acute asthma. Chest 98:1095–1098
Hulme EC, Birdsall NJM, Buckley NJ (1990) Muscarinic receptor subtypes. Ann Rev Pharmacol Toxicol 30:633–673
Kanto J, Klotz U (1988) Pharmacokinetic implications for the clinical use of atropine, scopolamine and glycopyrrolate. Acta Anaesthesiol Scand 32:69–78
Kentala E, Salonen M, Kanto J (1990) Anticholinergic premedication in Finland 1988. Acta Anaesthesiol Scand 34:17–20
Martindale (1989) Antimuscarinic agents. In: Reynolds JEF (ed) Martindale The Extra Pharmacopoea, 29 edn. Pharmaceutical Press London, pp 532–533
Mirakhur RK (1979) Intravenous administration of glycopyrronium: effects on cardiac rate and rhythm. Anaesthesia 34:458–462
Schroeckenstein DC, Bush RK, Chervinsky P, Busse WW (1988) Twelve-hour bronchodilatation in asthma with a single aerosol dose of the anticholinergic compound glycopyrrolate. J Allgergy Clin Immunol 82:115–119
Slovis CM, Daniels GM, Wharton DR (1987) Intravenous use of glycopyrrolate in acute respiratory distress due to bronchospastic pulmonary disease. Ann Emerg Med 16:898–900
Walker FB, Kaiser DL, Kowal MB, Suratt PM (1987) Prolonged effect of inhaled glycopryrrolate in asthma. Chest 91:49–51
Author information
Authors and Affiliations
Additional information
Correspondence to H. Fuder at the above address
Rights and permissions
About this article
Cite this article
Fuder, H., Meincke, M. Glycopyrronium bromide blocks differentially responses mediated by muscarinic receptor subtypes. Naunyn-Schmiedeberg's Arch Pharmacol 347, 591–595 (1993). https://doi.org/10.1007/BF00166941
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00166941