Abstract
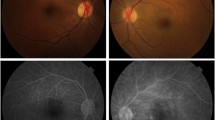
To obtain precise information on ophthalmological manifestations in patients with familial amyloidotic polyneuropathy (FAP), we performed ophthalmological and histopathological studies on 18 FAP patients and 6 asymptomatic individuals with a mutant transthyretin (TTR) gene. The incidence of vascular abnormalities of the conjunctiva and retina was surprisingly high in FAP patients. Abnormal conjunctival vessels were found mainly in the limbal area of FAP patients, but not in asymptomatic individuals with a mutant TTR gene. Conjunctival biopsy of 5 FAP patients and autopsy of another 2 FAP patients revealed that a significant amyloid deposit could be recognized in the superficial substantia propria of the conjunctiva and wall and perivascular area of the conjunctival vessels in all cases, a finding that is of diagnostic value. As for the retinal vessels, an abnormal arteriovenous ratio (A/V ratio), tortuous retinal vessels, cotton wool exudates and retinal hemorrhages were found in FAP patients. However, histopathological analysis of the retina in two autopsied cases revealed only a trace amount of amyloid deposit aruund the retinal vessels. Ophthalmological examination of three patients with pandysautonomia revealed that the appearance of both the conjunctival and retinal vessels of these patients was similar to that in FAP patients. These results indicate that in FAP patients ocular microangiopathy may be related to autonomic dysfunction as well as amyloid deposit.
Similar content being viewed by others
References
Araki S (1984) Type I familial amyloidotic polyneuropathy (Japanese type). Brain Dev 6:128–133
Cui J, Hayama M (1986) Phenol Congo red method as a new amyloid staining. Pathol Clin Med 4:1229–1232
Fall HF, Jackson J, Carey JH, Rukavina JG, Block WD (1982) Ocular manifestation of hereditary primary amyloidosis. Arch Ophthalmol 60:1036–1043
Goldberg MF, Payne JW, Brunt PW (1968) Ophthalmologic study of familial dysautonomia. Arch Ophthalmol 80:732–743
Ide M, Mita S, Ikegawa S, Maeda S, Shimada K, Araki S (1986) Identification of carriers of mutant prealbumin gene associated with familial amyloidotic polyneuropathy type I by southern blot procedures: study of six pedigrees in Arao destrict of Japan. Hum Genet 73:281–285
Inada K, Okamura R (1988) Localization of human prealbumin in ciliary epithelium and retina. Acta Soc Ophthalmol Jpn 92:741–745
Kaufman HE (1958) Primary familial amyloidosis. Arch Ophthalmol 60:1036–1043
Kuragi N, Yoshimoto H, Matsuyama S (1988) Microaneurysmlike vascular changes of the bulbar conjunctiva in diabetics. Folia Ophthalmol Jpn 39:855–859
Lee RL, Holze EA (1951) Peripheral vascular hemodynamics in the bulbar conjunctiva of subject with hypertensive vascular disease. J Clin Invest 30:539–546
Maruton RL, Herbert J, Dwork EA (1988) Transthyretin is synthesized in the mammalian eye. Biochem Res Commun 151:905–912
Mita S, Maeda S, Ide M, Tsuzuki T, Shimada K, Araki S (1986) Familial amyloidotic polyneuropathy diagnosed by cloned human prealbumin cDNA. Neurology 124:298–301
Murakami T, Yasuda Y, Mita S, Maeda S, Shimada K, Fujimoto T, Araki S (1991) Prealbumin gene expression during mouse development studies by in situ hybridization. Cell Differ Dev (in press)
Oonishi E (1960) Beiträge zum Studium der essentiellen Hypotension Studien besonders durch Ophthalmodynamometrie und Kalibermessung der Netzhautgefäße. Acta Soc Opthalmol Jpn 64:458–475
Sakai Y, Ando E, Maruoka S, Okamura R (1990) Vascular abnormalities (aneurysms) of the bulbar conjunctiva. 1. Studies on healthy subjects and diseased subjects. Ther Res 11:31–36
Savage DJ, Mango CA, Streeten BW (1982) Amyloidosis of the vitreous: fluorescein angiographic findings and association with neovascularization. Arch Ophthalmol 100:1776–1779
Spiro RG (1976) Search for a biochemical basis of diabetic microangiopathy. Diabetologia 12:1–14
Tawara S, Nakazato M, Kanagawa K, Maeda H, Araki S (1983) Identification of amyloid prealbumin variant in familial amyloidotic polyneuropathy (Japanese type). Biochem Biophys Res Commun 116:880–888
Tóth Z (1958) Was kann mit Hilfe des Augenspiegels am Fundus Hypertoniker festgestellt werden? Klin Monatsbl Augenheilkd 133:368–376
Vallat M, Vallat R, Lonbet R, Leboutel MJ (1976) Résultats de la biopsie conjunctivale dans lámylose géneralisée. J Fr Ophthalmol 2:275–278
Author information
Authors and Affiliations
Additional information
Offprint requests to: E. Ando
Rights and permissions
About this article
Cite this article
Ando, E., Ando, Y., Maruoka, S. et al. Ocular microangiopathy in familial amyloidotic polyneuropathy, type I. Graefe's Arch Clin Exp Ophthalmol 230, 1–5 (1992). https://doi.org/10.1007/BF00166754
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00166754