Summary
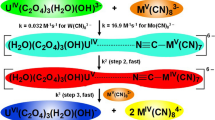
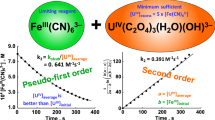
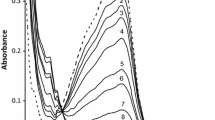
The reaction between hydroxopentaaquochromium(III) and octacyanomolybdate(IV) was investigated spectrophotometrically and obeyed a 2:1 reactant stoichiometry with respect to formation of the [Cr(H2O)4OH]2 Mo(CN)8 complex. Kinetic studies reveal that the reaction is first order with respect to hydroxopentaaquochromium(III) in the presence of an excess of octacyanomolybdate(IV). The reaction rate increased with an increase in the ionic strength and temperature, and decreased with an increase in hydrogen ion concentration. A mechanism has been proposed based upon ion-pair formation. The results are best accounted for by the Eigen-Tamm mechanism. Anation of [Cr(H2O)5OH]2+ is discussed in terms of an associative interchange (I a) where bond breaking and bond making are equally important. The activation parameters were calculated using Arrhenius's equation.
Similar content being viewed by others
References
C. Blanco, J. M. Hernado and M. Mateo, Can. J. Chem., 67, 1305 (1989).
M. Ardon and A. Linenberg, J. Phys. Chem., 65, 1443 (1961).
M. Thompson and R. E. Connick, Inorg. Chem., 20, 2279 (1981).
H. Stunzi and W. Marty, Inorg. Chem., 22, 2145 (1983).
H. Stunzi and W. Marty, Inorg. Chem., 23, 2160 (1984).
D. Rai, B. M. Sass and D. A. Moore, Inorg. Chem., 26, 345 (1987).
C. H. Langford, Inorg. Chem., 18, 3289 (1979).
C. H. Langford and H. Gray, Ligand Substitution Proceses, Benjamin, NY, 1965.
R. E. Hamm, R. L. Johnson, R. H. Perkins and R. E. Davis, J. Am. Chem. Soc., 80, 4469 (1958).
S. C. Tyagi and A. A. Khan, J. Inorg. Nucl. Chem., 41, 1447 (1979).
I. A. Khan, M. Shahid and Kabiruddin, J. Chem. Soc., Dalton Trans., 3007 (1990).
Kabiruddin, I. A. Khan and M. Shahid, Indian J. Chem., 32A, 171 (1993).
D. Banerjea and C. Chatterjee, J. Inorg. Nucl. Chem., 31, 3845 (1969).
D. Banerjea and S. D. Chaudhuri, J. Inorg. Nucl. Chem., 32, 1617 (1970).
D. Banerjea and P. Chaudhuri, J. Inorg. Nucl. Chem., 32, 2697 (1970).
J. N. Mandal and G. S. De, Indian J. Chem., 16A, 58 (1978).
J. N. Mandal and G. S. De, Indian J. Chem., 17A, 254 (1979).
S. I. Ali and S. Sharma, Indian J. Chem., 24A, 31 (1985).
S. I. Ali and Z. Murtaza, Polyhedron, 4, 463 (1985).
S. I. Ali, Z. Murtaza and A. A. Khan, Z. Physik. Chem. (Leipzig), 266, 1250 (1985).
J. G. Leipoldt, L. D. C. Bock and P. J. Colliers, Z. Inorg. Allg. Chem., 409, 343 (1974).
K. Emerson and W. M. Graven, J. Inorg. Nucl. Chem., 11, 309 (1959).
S. I. Ali and Z. Murtaza, Indian J. Chem., 23A, 258 (1984).
S. F. A. Kettle and R. V. Parish, Spectrochim. Acta, 21, 1087 (1965).
F. Basalo and R. G. Pearson, Mechanism of Inorganic Reactions, Wiley Eastern, New Delhi, 1977, p. 32.
B. K. Niogy and G. S. De, Proc. Indian Acad. Sci., 92, 153 (1983).
M. Eigen and K. Tamm, Z. Elektrochem, 66, 93, 107 (1962).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ali, S.I., Sharma, S. & Khan, Z. Kinetics and mechanism of the complexation between hydroxopentaaquochromium(III) and octacyanomolybdate(IV) ions in acidic medium. Transition Met Chem 21, 222–225 (1996). https://doi.org/10.1007/BF00165971
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00165971