Abstract
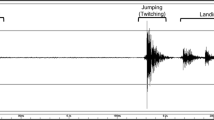
The jumping behavior of Strobilidium velox and Halteria grandinella was analyzed videographically. On average, undisturbed cells of these species jumped 1.7–3.6 and 8 times per minute and spent 0.8 and 1.0% of their time jumping, respectively. Both ciliate species initiated jumps after encounters with rotifer predators. S. velox jumped on contact with Asplanchna girodi, traveling a mean distance of 1.5 mm (33 body lengths) at a mean velocity of 7 mm/s (154 body lengths/s) at 17°C. H. grandinella jumped on contact or near contact with Synchaeta pectinata, traveling a mean distance of 0.37 mm (18 body lengths) at a mean velocity of 2.76 mm/s (131 body lengths/s) at 20°C. The maximum velocity recorded during these escape jumps was 16.07 mm/s for S. velox and 3.70 mm/s for H. grandinella. In S. velox, swimming velocity during jumps was not significantly correlated either with swimming velocity just before jumping (mean = 0.15 mm/s) or with distance traveled. In H. grandinella, jumping velocity and distance also were not significantly correlated. Jumping in S. velox and H. grandinella was calculated to require approximately 149% and 41 % of total metabolic rate, respectively. Jumping seemed to be an effective defense against rotifer predation. Only 3% of 93 S. velox cells contacted by A. girodi were captured, and only 12% of 92 H. grandinella cells contacted or closely approached by S. pectinata were captured; all other cells jumped away. A predation experiment showed that A. girodi was about twice as, and significantly more, likely to ingest Paramecium tetraurelia as S. velox in a mixture of equal numbers of these ciliates. The swimming velocity of S. velox during jumps is the highest one so far reported for an oligotrich, and equals the highest one reported for any ciliate (Mesodinium rubrum).
Similar content being viewed by others
References
Archbold JHG, Berger J (1985) A qualitative assessment of some metazoan predators of Halteria grandinella, a common freshwater ciliate. Hydrobiologia 126:97–102.
Bums CW, Gilbert JJ (1994) Predation on ciliates by freshwater calanoid copepods: rates of predation and relative vulnerabilities of prey. Freshwater Biol 30:377–393.
Crawford DW (1992) Metabolic cost of motility in planktonic protists: theoretical considerations on size scaling and swimming speed. Microb Ecol 24:1–10.
Crawford DW, Purdie DA (1992) Evidence for avoidance of flushing from an estuary by a planktonic, phototrophic ciliate. Mar Ecol Prog Ser 79:259–265.
Dale T (1987) Diel vertical distribution of planktonic ciliates in Lindåspollene, western Norway. Mar Microb Food Webs 2:15–28.
Fauré-Fremiet E (1948) Le rythme de marée du Strombidium oculatum Gruber. Bull Biol Fr Belg 82:3–23.
Fenchel T, Finlay BJ (1983) Respiration rates in heterotrophic, free-living protozoa. Microb Ecol 9:99–122.
Gilbert JJ (1985) Escape response of the rotifer Polyarthra: a high-speed cinematographic analysis. Oecologia 66:322–331.
Gilbert JJ (1987) The Polyarthra escape response: defense against interference from Daphnia. Hydrobiologia 147:235–238.
Gilbert JJ, Jack JD (1993) Rotifers as predators on small ciliates. Hydrobiologia 255/256:247–253.
Gilbert JJ, Stemberger RS (1985) Prey capture in the rotifer Asplanchna girodi. Verh Int Verein Limnol 22:2997–3000.
Gilbert JJ, Williamson CE (1978) Predator-prey behavior and its effect on rotifer survival in associations of Mesocyclops edax, Asplanchna girodi, Polyarthra vulgaris, and Keratella cochleari. Oecologia 37:13–22.
Jack JD, Gilbert JJ (1993) Susceptibilities of different-sized ciliates to direct suppression by small and large cladocerans. Freshwater Biol 29:19–29.
Jerome CA, Montagnes DJS, Taylor FJR (1993) The effect of the quantitative protargol stain and Lugol's and Bouin's fixatives on cell size: a more accurate estimate of ciliate species biomass. J Euk Microbiol 40:254–259.
Jonsson PR, Tiselius P (1990) Feeding behaviour, prey detection and capture efficiency of the copepod Acartia tonsa feeding on planktonic ciliates. Mar Ecol Prog Ser 60:35–44.
Kirk KL, Gilbert JJ (1988) Escape behavior of Polyarthra in response to artificial flow stimuli. Bull Mar Sci 43:551–560.
Klekowski RZ (1981) Size dependence of metabolism in protozoans. Verh Int Verein Limnol 21:1498–1502.
Lindholm T (1981) On the ecology of Mesodinium rubrum (Lohmann) (Ciliata) in a stagnant brackish basin on Åland, SW Finland. Kiel Meeresforsch 5:117–123.
Lindholm T (1985) Mesodinium rubrum—a unique photosynthetic ciliate. Adv Aquat Microbiol 3:1–48.
Maeda M (1986) An illustrated guide to the species of the families Halteriidae and Strobilididae (Oligotrichida, Ciliophora), free swimming protozoa common in the aquatic environment. Bull Ocean Res Inst Univ Tokyo 21:1–67.
Stemberger RS (1981) A general approach to the culture of planktonic rotifers. Can J Fish Aquat Sci 38:721–724.
Tamar H (1965) The culture, structure, and locomotion of Halteria grandinella. Acta Protozool 3:165–172.
Tamar H (1967) The movements and responses of Halteria grandinella. Acta Protozool 4:365–381.
Tamar H (1968) Observations on Halteria bifurcata sp. n. and Halteria grandinella. Acta Protozool 6:175–183.
Tamar H (1974) Further studies on Halteria. Acta Protozool 13:177–190.
Tamar H (1979) The movements of jumping ciliates. Archiv fur Protistenk 122:290–327.
Taylor FJR, Blackbourn DJ, Blackbourn J (1971) The red-water ciliate Mesodinium rubrum and its incomplete symbionts: a review including new ultrastructural observations. J Fish Res Bd Can 28:391–407.
Wickham SA, Gilbert JJ (1993) The comparative importance of competition and predation by Daphnia on ciliated protists. Arch Hydrobiol 126:289–313.
Wickham SA, Gilbert JJ, Berninger U-G (1993) Effects of rotifers and ciliates on the growth and survival of Daphnia. J Plankton Res 15:317–334.
Wurdak E, Clément P, Amsellem J (1983) Sensory receptors involved in the feeding behaviour of the rotifer Asplanchna brightwelli. Hydrobiologia 104:203–212.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gilbert, J. Jumping behavior in the oligotrich ciliates Strobilidium velox and Halteria grandinella, and its significance as a defense against rotifer predators. Microb Ecol 27, 189–200 (1994). https://doi.org/10.1007/BF00165817
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00165817