Summary
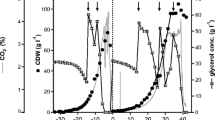
Alcohol oxidase biosynthesis was induced when Pichia pastoris was grown in a medium containing methanol as the sole carbon and energy source. Specific activity was highest during the logarithmic phase of growth (1.22 g acetaldehyde produced/g cell dry wt. per hour), and declined steadily thereafter. The addition of 0.1% (w/v) yeast extract to the methanol growth medium promoted higher biomass production, increased alcohol oxidase specific activity, and contributed to increased enzyme stability under use conditions. When P. pastoris was used for wholecell bioconversions, 30.2 g of ethanol were oxidized to 28 g acetaldehyde in 12 h, at a carbon recovery of 97%. Acetaldehyde concentrations in excess of 1 M were achieved when the concentration of the TRIS buffer, used to chemically trap the acetaldehyde, was increased to 1 M.
Similar content being viewed by others
References
Armstrong DW, Martin SM, Yamazaki H (1984a) Production of acetaldehyde from ethanol by Candida utilis. Biotechnol Lett 6:183–188
Armstrong DW, Martin SM, Yamazaki H (1984b) Production of ethyl acetate from dilute ethanol solutions by Candida utilis. Biotechnol Bioeng 26:1038–1041
Bormann C, Sahm H (1981) Degradation of peroxisomes in the methanol utilizing yeast Candida boidinii. In: Stewart GG, Russell I (eds) Advances in biotechnology. Pergamon Press, Toronto, p 465
Couderc R, Baratti J (1980) Oxidation of methanol by the yeast, Pichia pastoris. Purification and properties of the alcohol oxidase. Agric Biol Chem 44:2279–2289
Dijken JP van, Harder W (1974) Optimal conditions for the enrichment and isolation of methanol-assimilating yeasts. J Gen Microbiol 84:409–411
Dijkhuizen L, Hansen TO, Harder W (1985) Methanol, a potential feedstock for biotechnological processes. Trends Biotechnol 3:262–267
Duff SJB, Murray WD (1989) Oxidation of benzyl alcohol by whole cells of Pichia pastoris and by alcohol oxidase in aqueous and nonaqueous reaction media. Biotechnol Bioeng 34:153–159
Hitzman DO (1983) A process for decreasing the amount of dissolved oxygen in an aqueous fluid. European patent no. 0071990 A3
Holzer H (1976) Catabolite inactivation in yeast. Trends Biochem Sci 1:176–181
Hopkins TR (1984) Alcohol removal from blood with alcohol oxidase. US patent no. 4 450 153
Hopkins TR (1984) Alcohol rxidase from Pichia-type yeast. US patent no. 4 619 898
Kierstan M (1982) The enzymatic conversion of ethanol to acetaldehyde as model system. Biotechnol Bioeng 24:2275–2277
Murray WD, Duff SJB, Lanthier PH, Armstrong DW, Welsh FW, Williams RE (1988) Development of biotechnological processes for the production of natural flavors and fragrances. Dev Food Sci 17:1–18
Pilat P, Prokop P (1975) The effect of methanol, formaldehyde, and formic acid on growth of Candida boidini 11 Bh. Biotechnol Bioeng 17:1717–1728
Ramadan EM, Hazu W (1983) Effect of some elements and vitamins on the methanol oxidising yeast Pichia pastoris CBS 704. Egypt J Microbiol Special Issue:67–75
Raymond WR (1984) Process for the generation of acetaldehyde from ethanol. US patent no. 4 481 292
Veenhuis M, Dijken JP van, Harder W (1980) In vivo inactivation of alcohol oxidase (E.C. 1.2.3.1) in the yeast Hansenula polymorpha. Electron Microsc 2:84–85
Veenhuis M, Dijken JP van, harder W (1983) The significance of peroxomomes in the metabolism of one-carbon compounds in yeasts. Adv Microb Physiol 24:1–82
Volfova O, Pilat P (1974) Studies on methanol-oxidizing yeasts 1. Isolation and growth studies. Folia Microbiol 19:249–256
Author information
Authors and Affiliations
Additional information
Issued as NRCC no. 30256
Offprint requests to: W. D. Murray
Rights and permissions
About this article
Cite this article
Murray, W.D., Duff, S.J.B. & Lanthier, P.H. Induction and stability of alcohol oxidase in the methylotrophic yeast Pichia pastoris . Appl Microbiol Biotechnol 32, 95–100 (1989). https://doi.org/10.1007/BF00164829
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00164829