Abstract
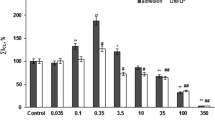
We investigated the effect of polymorphonuclear leukocyte (PMN)-generated oxygen metabolites on the ciliary beat frequency. PMNs were incubated with human respiratory cilia obtained by nasal brushing. The oxidative metabolism was stimulated by opsonized zymosan, and ciliary beat frequency was evaluated before and after activation of PMNs. Ciliary beat frequency was studied using video microscopy. Our results demonstrate a significant decrease in ciliary beat frequency after activation of PMNs. This effect was reduced by catalase. These data suggest that the PMN-generated oxygen metabolites, particulary H2O2, decrease beat frequency of human respiratory cilia.
Similar content being viewed by others
References
Amitani R, Wilson R, Rutman A, Read R, Ward C, Burnett D, Stockley RA, Cole PJ (1991) Effects of human neutrophil elastase and pseudomonas aeruginosa proteinases on human respiratory epithelium. Am J Respir Cell Mol Biol 4:26–32
Babior BM (1991) The respiratory burst oxidase and the molecular basis of chronic granulomatous disease. Am J Hematol 37:263–266
Bilton D, Leung AYT, Hill SL, Stockley RA (1992) Defective lung defences. Eur Respir Rev 2:159–165
Braga PC (1990) A variable-thickness, multipurpose culture chamber for high-magnification observation. J Microsc 159:285–288
Braga PC, Bossi R, Allegra L (1986) A method for maintaining, in vitro TV-monitoring, and counting ciliary beat frequency of samples from human ciliated respiratory epithelium brushing. Meth Find Exp Clin Pharmacol 8:321–326
Buchdahl RM, Reiser J, Ingram D, Rutman A, Cole PJ, Warner JO (1988) Ciliary abnormalities in respiratory disease. Arch Dis Child 63:238–243
Burman WJ, Martin WJ (1986) Oxidant-mediated ciliary dysfunction. Possible role in airway disease. Chest 89:410–413
Carson JL, Collier AM (1988) Ciliary defects: cell biology and clinical perspectives. Adv Pediatr 35:139–165
Claster S, Chiu DT, Quintanilha A, Lubin B (1984) Neutrophils mediate lipid peroxidation in human red cells. Blood 64:1079–1084
Cochrane CG (1991) Cellular injury by oxidants. Am J Med 91:23S–30S
Cochrane CG (1992) Mechanisms of cell damage by oxidants. In: Jesaitis AJ, Dratz EA (eds) Molecular Basis of Oxidative Damage by Leukocytes. CRC Press, Boca Raton, Florida, pp 149–162
Cross CE, Halliwell B, Allen A (1984) Antioxidant protection: a function of tracheobronchial and gastrointestinal mucus. Lancet 1:1328–1330
Ganbo T, Hisamatsu K, Nakazawa T, Kamijo A, Murakami Y (1991) Platelet activating factor (PAF) effects on ciliary activity of human paranasal sinus mucosa in vitro. Rhinology 29:231–237
Gecha OM, Fagan JM (1992) Protective effect of ascorbic acid on the breakdown of proteins exposed to hydrogen peroxide in chicken skeletal muscle. J Nutr 122:2087–2093
Giorgi PL, Oggiano N, Kantar A, Fabbrizi E, Piatti G, Braga PC (1991) Analysis of respiratory cilia motion and beat frequency counting in screening for primary ciliary dyskinesia in pediatric age. Riv Ital Pediatr 17:707–711
Giorgi PL, Oggiano N, Braga PC, Catassi C, Gabrielli O, Coppa GV, Kantar A (1992) Cilia in children with recurrent upper respiratory tract infections: ultrastructural observations. Pediatr Pulmonol 14:201–205
Halliwell B (1991) Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am J Med 91:145–225
Hastie AT, Loegering DA, Gleich GJ, Kueppers F (1987) The effect of purified human eosinophil major basic protein on mammalian ciliary activity. Am Rev Respir Dis 135:848–853
Hinshaw DB, Burger JM, Armstrong BC, Hyslop PA (1989) Mechanism of endothelial cell shape change in oxidant injury. J Surg Res 46:339–349
Jackowski JT, Szepfalusi ZS, Wanner DA, Seybold ZS, Sielczak MW, Lauredo IT, Adams T, Abraham WM, Wanner A (1991) Effects of P. aeruginosa-derived bacterial products on tracheal ciliary function: role of O2 radicals. Am J Physiol 260:L61-L67
Jesaitis AJ, Allen RA (1988) Activation of the neutrophil respiratory burst by chemoattractants: regulation of the N-formyl peptide receptor in the plasma membrane. J Bioenerg Biomembr 20:679–707
Kantar A, Wilkins G, Swoboda B, Littarru GP, Bertoli E, Catassi C, Coppa G, Giorgi PL (1990) Alterations of the respiratory burst of polymorphonuclear leukocytes from diabetic children. Acta Paediatr Scand 79:535–541
Kantar A, Oggiano N, Romagnoni GG, Giorgi PL (1991) Effect of oral administration of bacterial extracts on the bactericidal capacity of polymorphonuclear leucocytes in children with recurrent respiratory infections. J Intern Med Res 19:451–456
Kantar A, Giorgi PL, Curatola G, Fiorini R (1992) Changes in plasma membrane microheterogeneity of polymorphonuclear leukocytes during the activation of the respiratory burst. In: Jesaitis AJ, Dratz EA (eds) Molecular Basis of Oxidative Damage by Leukocytes. CRC Press, Boca Raton, Florida, pp 243–246
Khan AR, Bengtsson B, Laitinen LA (1990) Influence of H2O2 on the ciliary beat activity and contractile response of the guinea pig trachea (Abstract). Am Rev Respir Dis 141(suppl):A532
Kobayashi K, Salathe M, Pratt MM, Cartagena NJ, Soloni F, Seybold ZV, Wanner A (1992) Mechanism of hydrogen peroxide-induced inhibition of sheep airway cilia. Am J Respir Cell Mol Biol 6:667–673
McCord JM (1992) Superoxide production and human disease. In: Jesaitis AJ, Dratz EA (eds) Molecular Basis of Oxidative Damage by Leukocytes. CRC Press, Boca Raton, Florida, pp 225–239
Nieminen MM, Moilanen EK, Nyholm JEJ, Koskinen MO, Karvonen JI, Metsa-Ketela TJ, Vapaatalo H (1991) Platelet-activating factor impairs mucociliary transport and increases plasma leukotriene B4 in man. Eur Respir J 4:551–560
Olivero A, Miglietta A, Gadoni E, Gabriel L (1990) 4-Hydroxynonenal interacts with tubulin by reacting with its functional -SH groups. Cell Biochem Funct 8:99–105
Rutland J, Cole PJ (1980) Non-invasive sampling of nasal cilia for measurement of beat frequency and study of ultrastructure. Lancet ii:564–565
Schiff LJ, Eisenberg WC, Dziuba J, Taylor K, Moore SJ (1987) Cytotoxic effects of singlet oxygen. Environ Health Perspect 76:199–203
Sklar LA, McNeil VM, Jesaitis AJ, Painter RG, Cochrane CG (1982) A continuous, spectroscopic analysis of the kinetics of elastase secretion by neutrophils. The dependence of secretion upon receptor occupancy. J Biol Chem 257:5471–5475
Sleigh MA, Blake JR, Liron N (1988) The propulsion of mucus by cilia. Am Rev Respir Dis 137:726–741
Stommel EW, Stephens RE (1985) Cyclic AMP and calcium in the differential control of mytillus gill cilia. J Comp Physiol 157:451–459
Varani J, Fligiel SEG, Till GO, Kunkel RG, Ryan US, Ward PA (1985) Pulmonary endothelial cell killing by human neutrophils: possible involvement of hydroxyl radical. Lab Invest 53: 656–663
Ward PA (1991) Mechanisms of endothelial cell killing by H2O2 or products of activated neutrophils. Am J Med 91:89S–94S
Ward PA, Mulligan MS (1992) Leukocyte oxygen products and tissue damage. In Jesaitis AJ, Dratz EA (eds) Molecular Basis of Oxidative Damage by Leukocytes. CRC Press, Boca Raton, Florida, pp 139–147
Webber SE, Morikawa T, Widdicombe JG (1992) Platelet-activating factor relaxes ferret tracheal smooth muscle and reduces transepithelial potential difference in vitro. Br J Pharmacol 105:223–229
Webber SE, Morikawa T, Widdicombe JG (1992) PAF-induced muscarinic cholinoceptor hyperresponsiveness of ferret tracheal smooth muscle and gland secretion in vitro. Br J Pharmacol 105:230–237
Weisman Z, Fink A, Alon A, Poliak Z, Tabachnik E, Priscu L, Bentwich Z (1990) Leukotriene C4 decreases the activity of respiratory cilia in vitro. Clin Exp Allergy 20:389–393
Weiss SJ, LoBuglio AF (1982) Biology of disease: phagocyte-generated oxygen metabolites and cellular injury. Lab Invest 47:5–18
Wilson R, Roberts D, Cole P (1985) Effect of bacterial products on human ciliary function in vitro. Thorax 40:125–131
Author information
Authors and Affiliations
Additional information
Offprints requests to: A. Kantar
Rights and permissions
About this article
Cite this article
Kantar, A., Oggiano, N., Giorgi, P.L. et al. Polymorphonuclear leukocyte-generated oxygen metabolites decrease beat frequency of human respiratory cilia. Lung 172, 215–222 (1994). https://doi.org/10.1007/BF00164438
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00164438