Summary
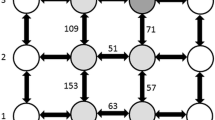
Leptogenys processionalis Jerdon forages on termites and other arthropods by raiding in branched trails. Growth and topology of these search trails were studied using Horton's (1945) technique orginally developed to analyze the branching pattern of river systems. Branching was always a bifurcating process and branches emerged symmetrically on either side of the main trail. Branching coefficients (R b ) were similar to those of a few biological branching systems, such as lungs, that are considered to be non-random in their branching pattern. The R b values indicated that the rate of branching and growth of trails remained constant within each foraging bout. The length of trails became shorter as they grew out and branched. The branching process was a function of the spatial separation of food patches in the terminal search field. Ants in the terminal search field send signals on encountering prey. The recruits cannot discriminate between these signals if they arise from two food patches situated <40 cm from each other, and hence converge on them in a single trail. However, discrimination is possible when food patches are >40 cm apart and hence recruits congregate on them separately in two trails, resulting in branching. Thus, the branching process is a result of independent decision by the ants conforming to certain simple rules and not a collective decision of the whole colony. We argue that mass recruiting ants selected to forage by branching pattern of trails because of its efficiency over other topologies (Stevens 1973) in minimizing the cost of travel, both from the nest to the food patches and between food patches. Further, the branch angles appear to be a trade-off to minimize travel cost and the resistance to the flow of ants comprising the column.
Similar content being viewed by others
References
Barker SB, Cummings G, Horsefield K (1973) Qualitative morphometry of the branching structures of trees. J Theor Biol 40:33–49
Batschelet E (1981) Circular statistics. Academic Press, New York
Bern MW, Graham RL (1989) The shortest-network problem. Sci Am 260:66–71
Chadab R, Rettenmeyer CW (1975) Mass recruitment by army ants. Science 188:1124–1125
Cheverton J (1982) Bumble bees may use a suboptimal arbitrary handedness to solve difficult foraging decisions. Anim Behav 30:934–935
Cody ML (1971) Finch flocks in the Mohave desert. Theor Popul Biol 2:142–158
D'Arcy Thompson W. (1942) On growth and form, 2nd edn. Cambridge University Press, Cambridge
Franks NR (1989) Army ants: a collective intelligence. Am Sci 77:138–145
Franks NR, Fletcher CR (1983) Spatial patterns in army ant foraging and migration: Eciton burchelli on Baro Colorado Island Panama. Behav Ecol Sociobiol 12:261–270
Ganeshaiah KN, Veena T (1988) Plant design and nonrandom foraging by ants on Croton bonplandianum Baill (Euphorbiaceac). Anim Behav 36:1683–1690
Haggett P, Chorley RJ (1969) Network analysis in geography. Arnold, London
Heinrich B (1979) Resource heterogenity and patterns of movement in foraging bumblebees. Oecologia 40:235–245
Holt SJ (1955) On the foraging activity of the wood ant. J Anim Ecol 24:1–34
Horsefield K (1980) Are diameter, length and branching ratios meaningful in the lung? J Theor Biol 87:773–784
Horton RE (1945) Erosional development of streams and their drainage basins: hydrophysical approach to quantitative morphology. Geol Soc Am Bull 56:275
Leopold LB (1971) Trees and streams: the efficiency of branching process. J Theor Biol 31:339
Levis DA, Kerster NW, Weidzlek M (1971) Pollinator flight directionality and its effect on pollen flow. Evolution 25:113–118
Maschwitz U, Schönegge P (1983) Forage communication, nest moving, recruitment and prey specailization in the oriental ponerine Leptogenys chinensis. Oecologia 57:175–182
Mirenda JT, Topoff H (1980) Nomadic behaviour or army ants in a desert-grassland habitat. Behav Ecol Sociobiol 7:129–135
Mirenda JT, Eakins DG, Gravelle K, Topoff H (1980) Predatory behaviour and prey selection by army ants in a desert-grassland habitat. Behav Ecol Sociobiol 7: 119–127
Moffett MW (1988) Foraging dynamics in the group-hunting myrmicine ant Pheidolegeton diversus. J Insect Behav 1:309–331
Murray CD (1926) The physiological principle of minimum work applied to the angle of branching of arteries. J Gen Physiol 9:835–841
Murray CD (1927) A relationship between circumference and weight in trees and its bearing on branching angles. J Gen Physiol 10: 725–739
Musthak Ali TM (1981) Ant fauna (Hymenoptera: Formicidae) of Banagalore with observations on their nesting and foraging habits. MSc (Agri) thesis. University of Agricultural Sciences, Bangalore
Park D (1985) Does Horton's law of branch length apply to open branching system? J Theor Biol 112:299–313
Pyke GH (1978a) Optimal foraging: movement patterns of bumble bees between inflorescences. Theor Popul Biol 133:72–98
Pyke GH (1978b) Are animals efficient harvesters? Anim Behav 26:241–250
Pyke GH (1978c) Optimal foraging in bumble bees and coevolution with their plants. Oecologia 36:281–283
Reyes JL (1986) Adaptability of foraging trails in Messor barbarus. Insectes Soc 33:249–257
Shivashankar T (1985) Studies on the ecology and foraging behaviour of Leptogenys processionalis Jerdon (Hymenoptera: Formicidae). MSc (Agri) thesis. Universitry of Agricultural Sciences, Bangalore
Stephens DW, Krebs JH (1986) Foraging theory. Princeton University Press, Princeton NJ
Stevens PS (1973) Space, architecture and biology. Syst Zool 22:405
Stevens PS (1974) Patterns in nature. Little Brown, Boston
Stahler AN (1952) Hypsometric (area altitude) analysis of erosional topology. Geol See Am Bull 63:1117
Taylor FW (1978) Foraging behaviour in ants: theoretical considerations. J Theor Biol 71: 542–563
Topoff H, Lawson L (1979) Orientation of the army ant Neivamyrmex nigrescens: integration of chemical and tactile information. Anim Behav 27:429–433
Trinci APJ (1973a) Growth of wild type and spreading colonial mutants of Neurospora crassa in both culture and agar medium. Arch Microbiol 91:113–126
Trinci APJ (1973b) The hyphal growth unit of wild and spreading colonial mutants of Neurospora crassa. Arch Microbiol 91:127–136
Veena T, Ganeshaiah KN (1991) Non random search pattern of ants foraging on honeydew of aphids in cashew inflorescences. Anim Behav 41:7–15
Zimmerman M (1979) Optimal foraging: a case for random movement. Oecologia 43:261–267
Zimmerman M (1981) Optimal foraging, plant density and the marginal value theorem. Oecologia 49:148–153
Zimmerman M (1982) Optimal foraging: random movement by pollen collecting bumblebees. Oecologia 53:394–398
Author information
Authors and Affiliations
Additional information
Offprint requests to: T. Veena
Rights and permissions
About this article
Cite this article
Ganeshaiahl, K.N., Veena, T. Topology of the foraging trails of Leptogenys processionalis — why are they branched?. Behav Ecol Sociobiol 29, 263–270 (1991). https://doi.org/10.1007/BF00163983
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00163983