Summary
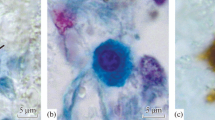
Mast cells have been described extensively in rodents and humans but not in pigs, and the objective of this study was to characterize porcine mast cells by histochemistry and electron microscopy. Carnoy's fluid proved to be a good fixative but fixation with neutral buffered formalin blocked staining of most mast cells. Alcian Blue stained more mast cells than did Toluidine Blue (pH 0.5), although Alcian Blue also stained goblet cells. In pigs, unlike rodents, the Alcian Blue method did not distinguish between mast cells in the intestinal mucosa and those in the connective tissue of the intestinal submucosa, tongue and skin. Mast cells were significantly larger in adult pigs than in piglets; in adult pigs and piglets, mast cells in the intestinal mucosa were significantly larger than those in submucosal connective tissue, and they were more varied in shape in piglets and adults. Granules in mast cells in the intestinal mucosa stained less intensely than those in mast cells in connective tissue of tongue, skin and intestinal submucosa. Mast cells in the connective tissue of the tongue, skin and intestinal submucosa fluoresced strongly when stained with berberine sulphate or with a mixture of berberine sulphate and Acridine Orange, but mast cells in the intestinal mucosa did not. All mast cells reacted positively in an enzyme-histochemical method previously used to detect human tryptase but not in a method previously used to detect human chymase. Mast cells in the medulla of thymus stained similarly to mast cells in the intestinal mucosa. Ultrastructural differences between mast cells were not detected.
Similar content being viewed by others
References
Ashraf, M., Urban, J. F. & Lee, T. D. G. (1988) Characterization of isolated porcine intestinal mucosal mast cells following infection with Ascaris suum.Vet. Parasitol. 29, 143–58.
Burnet, F. M. (1965) Mast cells in the thymus of NZB mice. J. Path. Bact. 89, 271–84.
Caulfield, J. P., Lewis, R. A., Hein, A. & Austen, K. F. (1980) Secretion of dissociated human pulmonary mast cells: evidence for solubilization of granule contents before discharge. J. Cell Biol. 85, 299–312.
Chen, W., Alley, M. R., Manktelow, B. W. & Slack, P. (1990) Mast cells in the bovine lower respiratory tract: morphology, density and distribution. Br. Vet. J. 146, 425–36.
Combs, J. W., Lagunoff, D. & Benditt, E. P. (1965) Differentiation and proliferation of embryonic mast cells of the rat. J. Cell Biol. 25, 577–92.
Craig, S. S., Schechter, N. M. & Schwartz, L. B. (1988) Ultrastructural analysis of human T and TC mast cells identified by immunoelectron microscopy. Lab. Invest. 58, 682–91.
Craig, S. S., Schechter, N. M. & Schwartz, L. B. (1989) Ultrastructural analysis of maturing human T and TC mast cells in situ. Lab. Invest. 60, 147–57.
Csaba, G. (1960) The human thymus gland: the problem of the thymus mast cells. Acta Biol. Acad. Sci. 9, 285–9.
Dimlich, V. W., Meineke, H. A., Reilly, F. D. & Mccluskey, R. S. (1980) The fluorescent staining of heparin in mast cells using berberine sulphate: compatibility with paraformaldehyde or o-phthalaldehyde induced fluorescence and metachromasia. Stain Technol. 55, 217–23.
Enerback, L. (1966a) Mast cells in rat gastrointestinal mucosa. 1. Effects of fixation. Acta Pathol. Microbiol. Scand. 66, 289–302.
Enerback, L. (1966b) Mast cells in rat gastrointestinal mucosa. 2. Dye-binding and metachromatic properties. Acta Pathol. Microbiol. Scand. 66, 303–12.
Enerback, L. (1966c) Mast cells in rat gastrointestinal mucosa. 3. Reactivity towards compound 48/80. Acta Pathol. Microbiol. Scand. 66, 313–32.
Enerback, L. (1974) Berberine sulphate binding to mast cell polyions: a cytofluormetric method for the quantitation of heparin. Histochemistry 42, 301–13.
Galli, S. J. (1990) New insights into ‘the riddle of the mast cells’: microenvironmental regulation of mast cell development and phenotypic heterogeneity. Lab. Invest. 62, 5–33.
Haensly, W. E. (1975) Thymus mast cell population in the domestic cat from two months to seven and one-half years. Southwest Veterinarian 28, 27–33.
Harvrima, I. T., Naukkarinen, A., Harvrima, R. J. & Fraki, J. E. (1988) Immunoperoxidase and enzyme-histochemical demonstration of human skin tryptase in cutaneous mast cells in normal and mastocytoma skin. Arch. Dermatol. Res. 280, 363–70.
Harvrima, I. T., Naukkarinen, A., Harvrima, R. J., Aalto, M. L., Neittaanmaki, H. & Horsmanheimo, M. L. (1990) Quantitative enzyme-histochemical analysis of tryptase- and chymase-containing mast cells in psoriatic skin. Arch. Dermatol. Res. 282, 428–33.
Jarrett, E. E. E. & Haig, D. M. (1984) Mucosal mast cells in vivo and in vitro. Immunology Today 5, 115–9.
Keisall, M. A. & Crabbe, E. D. (1952) Increased mast cells in the thymus of X-irradiated hamsters. Science 115, 123–4.
Marx, L., Hirota, M., Printup, C. A., Warnick, M. A. & Marx, W. (1960) Effects of exposure to 5°C and advancing age on tissue mast cells and heparin in male rats. Am. J. Physiol. 198, 180–2.
Osman, I. A. R., Garrett, J. R. & Smith, R. E. (1989) Enzyme histochemical discrimination between tryptase and chymase in mast cells of human gut. J. Histochem Cytochem. 37, 415–21.
Pabst, P. & Beil, B. (1989) Mast cell heterogeneity in the small intestine of normal, gnotobiotic and parasitized pigs. Int. Arch. Allergy Appl. Immunol. 88, 363–6.
Pipkorn, M., Karlsson, G. & Enerback, L. (1988) Phenotypic expression of proteoglycan in mast cells of the human nasal mucosa. Histochem. J. 20, 519–25.
Spicer, S. S. (1966) A correlative study of the histochemical properties of rodent acid mucopolysaccharides. J. Histochem. Cytochem. 8, 16–35.
Strobel, S., Miller, H. R. P. & Ferguson, A. (1981) Human intestinal mucosal mast cells: evaluation of fixation and staining techniques. J. Clin. Pathol. 34, 851–8.
Weidner, N. & Austen, K. F. (1990) Evidence for morphologic diversity of human mast cells. An ultrastructural study of mast cells from multiple body sites. Lab. Invest. 63, 63–72.
Wingren, M. & Enerback, L. (1983) Mucosal mast cell of the rat intestine: a re-evaluation of fixation and staining properties, with special reference to protein blocking and solubility of the granular glycosaminoglycan. Histochem. J. 15, 571–82.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Xu, L.R., Carr, M.M., Bland, A.P. et al. Histochemistry and morphology of porcine mast cells. Histochem J 25, 516–522 (1993). https://doi.org/10.1007/BF00159288
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00159288