Summary
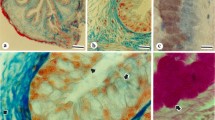
Immunohistochemical localizations of carbonic anhydrase isozymes (CA-I, CA-II and CA-III) in equine and bovine digestive tracts were studied. In the horse, epithelial cells in both the oesophagus and non-glandular part of the stomach lacked all three isozymes. In contrast, surface epithelial and parietal cells in the glandular region of the stomach showed reactivity for CA-II. In the small intestine, absorptive columnar cells covering the villi in the duodenum were positive for CA-II. The epithelium of the jejunum and ileum lacked all three isozymes. In the large intestine, CA-II was detected in the columnar cells in the upper part of the crypt. In cattle, epithelial cells of the oesophagus showed reactions for CA-I and CA-III but not for CA-II. Although the absorptive epithelial cells of the small intestine lacked CA-I, CA-II and CA-III, those of the upper part of large intestine crypts were heavily stained for all three isozymes.
Similar content being viewed by others
References
Aafjes, J. H. Carbonic anhydrase in the wall of the forestomach of cows. Br. Vet. J. 123 252–55
Argenzio, R. A. (1984) Introduction to Gastrointestinal. In Duke's Physiology of Domestic Animals. Digestion, Absorption and Metabolism function (edited by Swenson, M. J.). pp. 290–310.
Armstrong, J. M., Mayers, D. V., Verpoorte, J. A. & Edsall, J. T. (1966) Purification and properties of human erythrocyte carbonic anhydrase. J. Biol. Chem. 241, 5137–49.
Asari, M., Sasaki, K., KanoY. & Nishita, T. (1989) Immunohistochemical localization of carbonic anhydrase isozymes I, II and III in the bovine salivary glands and stomach. Arch. Histol. Cytol. 52, 337–44.
Asari, M., Sasaki, K., Kano, Y. & Nishita, T. (1991) Immunohis-tolocalization of the carbonic anhydrase isozymes I, II and III in equine salivary glands. Okajimas Folia Anat. Jap. 67, 467–72.
Carpentier, P., Chetail, M. & Fournie, J. (1985) Carbonic anhydrase in the rat salivary glands: distribution and ultrastructural localization. Biol. Cell. 54, 241–50.
Carter, M. J. & Parsons, D. S. (1970) The purification and properties of carbonic anhydrase from guinea-pig erythrocytes and mucosae of the gastrointestinal tract. Biochem. J. 120, 797–808.
Carter, M. J. (1971) The carbonic anhydrase in the rumen epithelial tissue of the ox. Biochem. Biophys. Acta 235, 222–36.
Carter, N. D., Hewett-Emmett, D., Jeffery, S. & Tashian, R. E. (1981) Testosterone-induced, sulfonamide-resistant carbonic anhydrase isozyme of rat liver is indistinguishable from skeletal muscle carbonic anhydrase. Fed. Eur. Biochem. Soc. Lett. 128, 114–8.
DeutschH. F. (1987) Carbonic anhydrase. Int. J. Biochem. 19, 101–13.
Deutsch, H. F., Funakoshi, S., Fujita, T., Taniguchi, N. & Hirai, H. (1972) Isolation in crystalline form and properties of six horse erythrocyte carbonic anhydrases. J. Biol. Chem. 247, 4499–502.
Engberg, P., Millquist, E., Pohl, G. & Lindskog, S. (1985) Purification and some properties of carbonic anhydrase from bovine skeletal muscle. Arch. Biochem. Biophys. 241, 628–38.
Funakoshi, S. & Deutsch, H. F. (1971) Human carbonic anhydrase. VI levels of isozymes in old and younger erythrocytes and in various tissues. J. Biol. Chem. 246, 1088–92.
Galfi, P., Kutas, F. & Neogrady, S. (1982) Immunohistochemical detection of bovine ruminal carbonic anhydrase isoenzyme. Histochemistry 74, 577–84.
Gros, G. & Dodgson, S. J. (1988) Velocity of CO2 exchange in muscle and liver. Ann. Rev. Physiol. 50, 669–94.
Hennigar, R. A., Schulte, B. A. & Spicer, S. S. (1983) Immunolo-calization of carbonic anhydrase isozymes in rat and mouse salivary and exorbital lacrimal glands. Anat. Rec. 207, 605–14.
Igarashi, S., KanoY., Nishita, T., Amasaki, H. & Asari, M. (1992) Comparison of the distribution of carbonic anhydrase isozymes (CA-I, CA-II, CA-III) in the rat gastrointestinal tract. J. Vet. Med. Sci. 54, 535–9.
IkejimaT. & Ito, S. (1984) Carbonic anhydrase in mouse salivary glands and saliva: a histochemical, immunohistochemical, and enzyme activity study. J. Histochem. Cytochem. 32, 625–35.
Jacobson, E. D. (1978) The Gastrointestinal System: Essentials of Human Physiology (edited by Rase, G.). pp. 370–443. Chicago Year Book Medical Publ.
Jeffery, S. (1991) Immunological Methods for Detection and Quantification of CA Isozymes In The Carbonic Anhydrases: Cellular Physiology and Molecular Genetic, (edited by Dodgson, S. J., Tashian, R. E., Gros, G. & Carter, N. D.). pp. 153–9.
JefferyS., Carter, N. D. & SmithA. (1986) Immunocytochemical localization of carbonic anhydrase isozymes I, II and III in rat skeletal muscle. J. Histochem. Cytochem. 34, 513–6.
Karhukorpi, E-K. (1991) Carbonic anhydrase II in rat acid secreting cells: comparison of osteoclasts with gastric parietal cells and kidney intercalated cells. Acta Histochem. 90, 11–20.
Kumpulainen, T. (1979) Immunohistochemical localization of human carbonic anhydrase isozymes C. Histochemistry 62, 271–80.
Leblond, C. P. (1965) The time dimension in histology. Am J. Anat. 116, 1–28.
Nishita, T. & Deutsch, H. F. (1981) Isolation of equine muscle carbonic anhydrase in crystalline form. Biochem. Biophys. Res. Commun. 103, 573–80.
Nishita, T., Matsushita, H. & Kai, M. (1987) Immunocytochemical localization of carbonic anhydrase III in equine skeletal muscle. Equine Vet. J. 19, 509–13.
Nishita, T., Oshige, H., Matsushita, H., Kano, Y. & Asari, M. (1989) The immunohistolocalization of carbonic anhydrase III in the submandibular gland of rats and hamsters. Histochemical J. 21, 8–14.
Nishita, T., Kinoshita, C., Maegaki, M. & Asari, M. (1990) Immunohistochemical studies of the carbonic anhydrase isozymes in the bovine placenta. Placenta 11, 329–36.
Register, A. M., Koester, M. K. & Noltman, E. A. (1978) Discovery of carbonic anhydrase in rabbit skeletal muscle and evidence for its identity with basic muscle protein. J. Biol. Chem. 253, 4143–52.
Shima, K., Tashiro, K., Hibi, N., Tsukada, Y. & Hirai, H. (1984) Carbonic anhydrase III: immunohistochemical localization of the carbonic anhydrase isozymes for their function in normal and pathologic cells. Ann. N.Y. Acad. Sci. 429, 382–97.
Spicer, S. S., Stoward, P. J. & Tashian, R. E. (1979) The immunohistolocalization of carbonic anhydrase in rodent tissue. J. Histochem. Cytochem. 27, 820–31.
Spicer, S. S., Ge, Z-H., Tashian, R. E., Hazen-Martin, D. J. & Schultz, B. A. (1990) Comparative distribution of carbonic anhydrase isozymes III and II in rodent tissues. Am. J. Anat. 187, 55–64.
Swenson, E. R. (1991) Distribution and functions of carbonic anhydrase in the gastrointestinal tract. In The Carbonic Anhydrases: Cellular Physiology and Molecular Genetics (edited by Dodgson, S. J., Tashian, R. E., Gros, G. & Carter, N. D.), pp. 265–87.
Tashian, R. E. (1989) The carbonic anhydrase: widening perspectives on their evolution, expression and function. BioEssays 10, 186–92.
Väänänen, H. K. & Autio-Harmainen, H. (1987) Carbonic anhydrase III: a new histochemical marker for myoepithelial cells. J. Histochem. Cytochem. 35, 683–6.
Väänänen, H. K., Carter, N. D. & Dodgson, S. J. (1991) Immuno-cytochemical localization of mitochondrial carbonic anhy drase in rat tissues. J. Histochem. Cytochem. 39, 451–9.
Wilbur, K. M. & Anderson, N. G. (1948) Electrometric and colorimetric determination of carbonic anhydrase. J. Biol. Chem. 176, 147–54.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sasaki, K., Igarashi, SI., Amasaki, T. et al. Comparative immunohistolocalization of carbonic anhydrase isozymes I, II and III in the equine and bovine digestive tract. Histochem J 25, 304–311 (1993). https://doi.org/10.1007/BF00159122
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00159122