Abstract
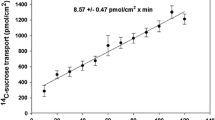
The uptake of selenite, selenate and selenomethionine (SeMet) was performed with brush border membrane vesicles (BBMV) prepared from rats fed selenium-deficient and supplemented diets. At equilibrium (60 min), the uptake of 75Se from [75Se]selenite ranged from 16.5 to 18.9 nmol mg-1 protein. There was a curvilinear relationship in the uptake of selenite over a concentration range of 10–1000 μ m. About 2 nmol mg-1 protein was obtained with selenomethionine (SeMet) which occurred between 90 and 180 s. In contrast to selenite, there was a linear relationship in the initial uptake of SeMet over a concentration range of 10–1000 μ m. The uptake of selenate was approximately 50-fold lower than selenite, reaching 350 pmol mg-1 protein. Dietary selenium level had no effect on the rate of 75Se accumulation by BBMV. Dramatic differences are found in the uptake and binding of selenium by BBMV incubated with different selenocompounds.
Similar content being viewed by others
References
American Institute of Nutrition. 1977 Report of the AIN Ad Hoc Committee on standards for nutritional studies. J Nutr 107, 1340–1348.
Arduser F, Wolffram S, Scharrer E. 1985 Active absorption of selenate by rat ileum. J Nutr 115, 1203–1208.
Arduser F, Wolffram S, Scharrer E, Schneider B. 1986 Transport of selenate and selenite across the brush border membrane of rat and sheep small intestine. Biol Trace Elem Res 9, 281–290.
Black RS, Tripp MJ, Whanger PD, Weswig PH. 1978 Selenium proteins in ovine tissues: III. Distribution of selenium and glutathione peroxidase in tissue cytosols. Bioinorg Chem 8, 161–172.
Brown DG, Burk RF, Seely RJ, Kiker KW. 1972 Effect of dietary selenium on the gastrointestinal absorption of 75SeO =3 in the rat. Int J Vit Nutr Res 42, 588–591.
Brown MW, Watkinson JH. 1977 An automated fluorimetric method for the determination of nanogram quantities of selenium. Anal Chim Acta 89, 29–35.
Dahlqvist A. 1968 Assay of intestinal disaccharidases. Anal Biochem 22, 99–107.
Duggleby RG. 1984 Regression analysis of nonlinear Arrhenius plots: an empirical model and a computer program. Comput Biol Med 14, 447–455.
Earle WR. 1943 Production of malignancy in vitro. IV. The mouse fibroblast culture and changes seen in living cells. J Natl Cancer Inst 4, 165–212.
Ganther HE, Corcoran C. 1969 Selenotrisulfides. II. Cross-linking of reduced pancreatic ribonuclease with selenium. Biochemistry 8, 2557–2568.
Hopfer U, Nelson K, Perrotto J, Isselbacher KJ. 1973 Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem 248, 25–32.
Humaloja T, Mykkanen H. 1986 Intestinal absorption of 75Selabeled sodium selenite and SeMet in chicks: effects of time, segment, selenium concentration and method of measurement. J Nutr 116, 142–148.
Kessler M, Toggenburger G. 1979 Nonelectrolyte transport in small intestinal membrane vesicles. The application of filtration for transport and binding studies. In: Carafoli E, Semenza G, eds. Membrane Biochemistry. A Laboratory Manual on Transport and Bioenergetics. New York: Springer-Verlag.
Langridge-Smith JE, Field M. 1981 Sulfate transport in rabbit ileum: characterization of the serosal border anion exchange process. J Membrane Biol 63, 207–214.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951 Protein measurement with the Folin phenol reagent. J Biol Chem 193, 265–275.
Lucke H, Stange G, Murer H. 1981 Sulfate-sodium cotransport by brush-border membrane vesicles isolated from rat ileum. Gastroenterology 80, 22–30.
McConnell KP, Cho GJ. 1965 Transmucosal movement of selenium. Am J Physiol 208, 1191–1195.
McConnell KP, Cho GJ. 1967 Active transport of l-SeMet in the intestine. Am J Physiol 213, 150–155.
Menard MP, Cousins RJ. 1983 Zinc transport by brush border membrane vesicles from rat intestine. J Nutr 113, 1434–1442.
Miller A, Bronner F. 1981 Calcium uptake in isolated brush border vesicles from rat small intestine. Biochem J 196, 391–401.
Muir WA, Hopfer U, King M. 1984 Iron transport across brush-border membranes from normal and iron-deficient mouse upper small intestine. J Biol Chem 259, 4896–4903.
Murer H, Kinne R. 1980 The use of isolated membrane vesicles to study epithelial transport processes. J Membrane Biol 55, 81–95.
Mykkanen HM, Wasserman RH. 1989 Uptake of 75Se-selenite by brush border membrane vesicles from chick duodenum stimulated by vitamin D. J Nutr 119, 242–247.
Paglia DE, Valentine WN. 1967 Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70, 158–169.
Smith PL, Orellana SA, Field M. 1981 Active sulfate absorption in rabbit ileum: dependence on sodium and chloride and effects of agents that alter chloride transport. J Membrane Biol 63, 199–206.
Steel RGD, Torrie JH. 1980 Principles and Procedures of Statistics, 2nd edn. New York: McGraw-Hill; 186–187.
Stevens BR, Wright SH, Hirayama BS, et al. 1982 Organic and inorganic solute transport in renal and intestinal membrane vesicles preserved in liquid nitrogen. Membrane Biochem 4, 271–282.
Vendeland SC, Butler JA, Whanger PD. 1992a Intestinal absorption of selenite, selenate and SeMet in the rat. J Nutr Biochem 3, 359–365.
Vendeland SC, Deagen JT, Whanger PD. 1992b Uptake of selenotrisulfides of glutathione and cysteine by brush border membranes from rat intestines. J Inorg Biochem 4, 131–140.
Wolffram S, Arduser F, Scharrer E. 1985 In vivo intestinal absorption of selenate and selenite by rats. J Nutr 115, 454–459.
Wolffram S, Anliker E, Scharrer E. 1986 Uptake of selenate and selenite by isolated intestinal brush border membrane vesicles from pig, sheep, and rat. Biol Trace Elem Res 10, 293–306.
Wolffram S, Grenacher B, Scharrer E. 1988 Transport of selenate and sulfate across the intestinal brush-border membrane of pig jejunum by two common mechanisms. Quart J Exp Physiol 73, 103–111.
Wolffram S, Berger B, Grenacher B, Scharrer E. 1989 Transport of selenoamino acids and their sulfur analogues across the intestinal brush border membrane of pigs. J Nutr 119, 706–712.
Wurmli R, Wolffram S, Stingelin Y, Scharrer E. 1989 Stimulation of mucosal uptake of selenium from selenite by l-cysteine in sheep small intestine. Biol Trace Elem Res 20, 75–85.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vendeland, S.C., Deagen, J.T., Butler, J.A. et al. Uptake of selenite, selenomethionine and selenate by brush border membrane vesicles isolated from rat small intestine. Biometals 7, 305–312 (1994). https://doi.org/10.1007/BF00144126
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00144126