Abstract
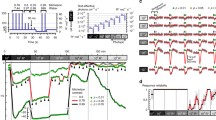
Extracellular ganglion cell responses were recorded to investigate mechanisms of light adaptation. Monochromatic test spots (575 nm) were projected onto the receptive field center of off-center cells and superimposed on a steady blue-green Ganzfeld background (Schott Filter BG 28), the strength of which was increased in steps of 0.5 log units to adapt rods. Response vs. log intensity functions were determined over a range of 7 log units of test light irradiance at each background level. At higher adaptation levels response thresholds followed the typical Weber function. Surprisingly at lower adaptation levels the sensitivity of the cell increased by about 0.7 log units, most markedly in a range of 1 log unit of moderate light adaptation when the background was changed from dark to the dimmest detectable background (10−5lm/m2). In the dark-adapted state a small off-response of long latency (40–100ms at 102 quanta · s−1 · μm−2) is observed at low rod stimulating test light irradiances. A transition to a cone-dominated transient response of 2 to 5 ms duration occurred at high intensities (105 quanta · s−1 · μm2). At mesopic levels the two responses seem to cancel each other, rendering a delayed off-response that is probably the result of rod-cone interaction. As in psychophysics, saturation can be observed at very high background intensities (106 quanta · s−1 μm−2). These data suggest interactions between rods and cones that determine the sensitivity of cat retinal ganglion cells at low levels of adaptation for suprathreshold stimuli.
Similar content being viewed by others
References
Witkovsky P. Excitation and adaptation in the vertebrate retina. Curr. Top. Eye 1980.
Ikeda M, Urakubo M. Rod-cone interrelation. J Opt Soc Am 1967; 59: 217–22.
Benimoff NI, Schneider S, Hood DC. Interactions between rod and cone channels above threshold: a test of various models. Vision Res 1982; 22: 1133–40.
Levine MW, Abramov I. An analysis of spatial summation in the receptive fields of goldfish retinal ganglion cells. Vision Res 1975; 15: 777–89.
Levine MW. Interactions between rod and cone channels: a model that includes inhibition. Vision Res 1984; 24: 513–6.
Levine MW, Frishman LJ, Enroth-Cugell C. Interactions between the rod and the cone pathways in the cat retina. Vision Res 1987; 27: 1093–104.
Baylor DA. Photoreceptor signals and vision. Invest Ophthalmol Vis Sci 1987; 28: 34–49.
Werblin FS. Control of retinal sensitivity, II: Lateral interactions at the outer plexiform layer. J Gen Physiol 1974; 63: 62–87.
Werblin FS, Copenhagen DR. Control of retinal sensitivity, III: Lateral interactions at the inner plexiform layer. J Gen Physiol 1974; 63: 88–110.
Normann RA, Werblin FA. Control of retinal sensitivity. J Gen Physiol 1974; 63: 37–61.
Goldberg SH, Frumkes TE, Nygaard RW. Inhibitory influence of unstimulated rods in the human retina: Evidence provided by examining cone flicker. Science 1983; 221: 180–2.
Alexander, KR, Fishman GA. Rod-cone interaction in flicker perimetry. Br J Ophthalmol 1984; 68: 303–9.
Alexander DR, Fishman GA. Rod influence on cone flicker detection: Variation with retinal eccentricity. Vision Res 1986; 26: 827–34.
Coletta NJ, Adams AJ. Adaptation of color-opponent mechanism increases parafoveal sensitivity to luminance flicker. Vision Res 1986; 26: 1241–8.
Drum B. Rod bleaches raise cone plateau thresholds. Invest Ophthalmol Vis Sci 1981; 20: (ARVO suppl.) 207.
Buck SL, Makous W. Rod-cone interaction on large and small backgrounds. Vision Res 1981; 21: 1181–7.
Stromeyer CF, Hill TL. The cone threshold: Spatial interactions of rod and cone adapting signals. Vision Res 1983; 23: 713–22.
Frumkes TE, Naarendorp F, Goldberg SH. The influence of cone adaptation upon rod-mediated flicker. Vision Res 1986; 26: 1167–76.
Arden GB, Frumkes TE. Stimulation of rods can increase cone flicker ERGs in man. Vision Res 1986; 26: 711–21.
Nelson R. Cat cones have rod input: A comparison of the response properties of cones and horizontal cell bodies in the retina of the cat. J Comp Neurol 1977; 172: 109–36.
Naarendorp F, Denny N, Frumkes TE. Rod light and dark adaptation influence conemediated spatial acuity. Vision Res 1988; 28: 67–74.
Levick WR. Interactions between rod and cone channels: A model that includes inhibition. Vision Res 1984; 24: 513–6.
Gouras P, Zrenner E. Enhancement of luminance flicker by color-opponent mechanisms. Science 1979; 205: 587–9.
Zrenner E. Neurophysiological aspects of color vision in primate. Monograph: Studies of Brain Function, Vol. 9. Berlin: Springer, 1983.
Enroth-Cugell C, Robson JG. The contrast sensitivity of retinal ganglion cells of the cat. J Gen Physiol 1966; 187: 517–76.
Olsen BT, Schneider T, Zrenner E. Characteristics of rod driven off-responses in cat ganglion cells. Vision Res 1986; 26: 835–45.
Aguilar M, Stiles WS. Saturation of the rod mechanism of the retina at high levels of stimulation. Opt Acta 1954; 1: 59–65.
Schneider T, Olsen BT, Zrenner E. Characteristics of the rod-cone transition in electroretinogram and optic nerve response. Clin Vision Sci 1987; 1: 81–91.
Rodieck RW, Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965; 28: 819–49.
Lützow A von, Wündsch L. Grenzen der zeitlichen Summation im Doppelreiz-ERG des Kaninchens. Vision Res 1967; 7: 565–71.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Günther, E., Zrenner, E. Rod and cone contribution to adaptation processes in cat retinal ganglion cells. Doc Ophthalmol 75, 83–95 (1990). https://doi.org/10.1007/BF00142597
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00142597