Summary
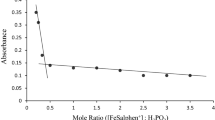
Nitrite ion reacts with bis(dihydrogentellurato) copper(III) through initial complex formation between the reactants in alkaline medium according to the equation
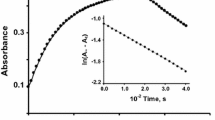
The rate increases with increase in [NaClO4]. The values of k and K are 1.60 × 10-4 and 9.55, respectively, at 25° C. The values of Δ 0H and Δ 0S associated with the equilibrium step are 25 kJ mol-1 and 102.7 J K-1 mol-1, whereas for the rate-determining step the values of ΔH ≠ and ΔS ≠ are 72.4k Jmol-1 and −74.3 JK-1mol-1, respectively.
Similar content being viewed by others
References
I. Rao, J. Hussain, S. K. Mishra and P. D. Sharma, J. Chem. Res. (S), 392 (1992).
S. K. Ghosh, R. N. Bose and E. S. Gould, Inorg. Chem., 26, 2688 (1987).
G. Stedman, Adv. Inorg. Chem., Radiochem., 22, 113 (1979).
W. K. Wilmarth, D. M. Stanbury, J. E. Byrd, H. N. Po and C. P. Chua, Coord. Chem. Rev., 51, 155 (1983).
T. A. Turney and G. A. Wright, Chem. Rev., 59, 497 (1959); T. A. Turney and G. A. Wright, J. Chem. Soc., 2415 (1958).
T. A. Turney, Oxidation Mechanisms, Butterworths, London, 1965, pp. 80, 143.
K. K. Sen Gupta and J. Karak, J. Chem. Res. (S), 258 (1989); K. K. Sen Gupta, J. Karak and A. Mahapatra, J. Chem. Res. (S), 366 (1989).
S. Chandra and K. L. Yadav, Telanta., 15, 349 (1968).
A. Balikungeri, M. Pelletier and D. Monnier, Inorg. Chim. Acta, 22, 7 (1977).
W. Levason and M. D. Spicer, Coord. Chem. Rev., 76, 45 (1987).
G. Svehla, Vogel's Textbook of Macro and Semimicro Qualitative Inorganic Analysis, Orient Longman, 1985, pp. 336, 31.
M. S. Ram and D. M. Stanbury, J. Am. Chem. Soc., 106, 8136 (1984).
N. N. Greenwood and A. Earnshaw, Chemistry of the Element, Pergamon Press, Oxford, 1984, p. 1379.
C. W. Davies, Ion Association, Butterworths, London, 1985, p. 39.
M. A. Frieberg and D. Meyerstein, J. Chem. Soc., Chem. Commun., 127 (1977).
W. A. Muale and D. Mayerstein, J. Chem. Soc., Chem. Commun., 893 (1979).
E. K. Barefield and M. C. Mocella, J. Am. Chem. Soc., 97, 4238 (1975).
D. C. Olson and J. Vasilevskis, Inorg. Chem., 10, 463 (1971).
G. P. Panigrahi and A. C. Pathy, Inorg. Chem., 23, 2133 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sen Gupta, K.K., Sanyal, A. Reduction of bis(dihydrogentellurato) copper(III) by nitrite ion in alkaline medium. Transition Met Chem 19, 329–331 (1994). https://doi.org/10.1007/BF00139105
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00139105