Summary
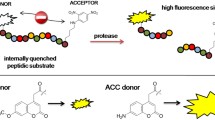
Two series of intramolecularly quenched fluorogenic oligopeptide substrates with the general sequences DABCYL-Lys-Phe-Gly-Gly-Ala-Xxx-EDANS and Abz-Lys-Phe-Gly-Gly-Ala-Xxx-Tyr(m-NO2)-NH2 have been used to explore the effect of P3′ substituents and donor/acceptor pairs on the kinetic parameters for papain-catalyzed hydrolysis. The steady-state constants are moderately affected by amino acid or fluorophore/quencher replacement. No correlation between the hydrophobicity of the P3′ substituent and the kinetic parameters was found.
Similar content being viewed by others
Abbreviations
- Abz:
-
anthranilic acid or anthranilamide
- Boc:
-
tert-butyloxycarbonyl
- BOP:
-
benzotriazolyloxy tris-(dimethylamino)-phosphonium hexafluorophosphate
- DABCYL:
-
4-(4-dimethylaminophenylazo)benzoic acid
- DCM:
-
dichloro-methane
- DIEA:
-
diisopropylethylamine
- DIPCDI:
-
diisopropylcarbodiimide
- DMF:
-
N,N-dimethylformamide
- DMSO:
-
dimethylsulfoxide
- E-64:
-
trans-epoxysuccinyl-l-leucylamido(4-guanidino)butane
- EDCI:
-
1-(3-dimethylaminopropyl)-3-ethylcarbodi-imide hydrochloride
- EDTA:
-
ethylenediaminetetraacetic acid
- EDANS:
-
5-[(2-aminoethyl)amino]naphthalene-1-sulfonic acid
- FABMS:
-
fast atom bombardment mass spectrometry
- Fmoc:
-
9-fluorenylmethyloxycarbonyl
- HOBt:
-
1-hydroxybenzotriazole
- HPLC:
-
high pressure liquid chromatography
- MBHA:
-
4-methylbenzhydrylamine (resin)
- MCA:
-
methylcoumarylamide
- Nle:
-
norleucine
- PAL:
-
tris(alkoxy)benzylamide linker
- Su:
-
succinimide
- TFA:
-
trifluoroacetic acid
- tR :
-
retention time
- Tyr(m-NO2):
-
meta-nitrotyrosine
- Z:
-
benzyloxycarbonyl
References
Schechter, I. and Berger, A., Biochem. Biophys. Res. Commun., 27 (1967) 157.
Compadre, C.M., Hansch, C., Klein, T.E. and Langridge, R., Biochim. Biophys. Acta, 1038 (1990) 158.
Stöcker, W., Ng, M. and Auld, D.S., Biochemistry, 29 (1990) 10418.
Margolin, N., Heath, W., Osborne, E., Lai, M. and Vlahos, C., Biochem. Biophys. Res. Commun., 167 (1990) 554.
Matayoshi, E.D., Wang, G.T., Krafft, G.A. and Erickson, J., Science, 247 (1990) 954.
Juliano, L., Chagas, J.R., Hirata, I.Y., Carmona, E., Sucupira, M., Oliveira, E., Oliveira, E.B. and Camargo, A.C.M., Biochem. Biophys. Res. Commun., 173 (1990) 647.
Meldal, M. and Breddam, K., Anal. Biochem., 195 (1991) 141.
Chagas, J.R., Juliano, L. and Prado, E.S., Anal. Biochem., 192 (1991) 419.
García-Echeverría, C., Kofron, J.L., Kuzmic, P., Kishore, V. and Rich, D.H., J. Am. Chem. Soc., 114 (1992) 2758.
Nishino, N., Makinose, Y. and Fujimoto, T., Chem. Lett., (1992) 77.
Maggiora, L.L., Smith, C.W. and Zhang, Z.-Y., J. Med. Chem., 35 (1992) 3727.
García-Echeverría, C., Kofron, J.L., Kuzmic, P. and Rich, D.H., Biochem. Biophys. Res. Commun., 191 (1993) 70.
Zhang, Z.-Y., Maclean, D., Thieme-Sefler, A.M., Roeske, R.W. and Dixon, J.E., Anal. Biochem., 211 (1993) 7.
García-Echeverría, C. and Rich, D.H., Biomed. Chem. Lett., 3 (1993) 1601.
Hirata, I.Y., Cezari, M.H.S., Nakaie, C.R., Boschcov, P., Ito, A.S., Juliano, M.A. and Juliano, L., Lett. Pept. Sci., 1 (1994) 299.
Peranteau, A.G., Kuzmic, P., Angell, Y., García-Echeverría, C. and Rich, D.H., Anal. Biochem., 227 (1995) 242.
Grøn, H., Meldal, M. and Breddan, K., Biochemistry, 31 (1992) 6011.
García-Echeverría, C. and Rich, D.H., FEBS Lett., 297 (1992) 100.
García-Echeverría, C. and Rich, D.H., Biochem. Biophys. Res. Commun., 187 (1992) 615.
Berger, A. and Schechter, I., Phil. Trans. R. Soc. London Ser. B., 257 (1970) 249.
Wang, G.T., Huffaker, J.A., Matayoshi, E.D. and Krafft, G.A., Tetrahedron Lett., 31 (1990) 6493.
Wang, G.T., Chung, C.C., Holzman, T.F. and Krafft, G.A., Anal. Biochem., 210 (1993) 351.
Albericio, F., Kneib-Cordonier, N., Biancalana, S., Gera, L., Masada, R.I., Hudson, D. and Barany, G., J. Org. Chem., 55 (1990) 3730.
Kaiser, E., Colescott, R.L., Bossinger, C.D. and Cook, P.I., Anal. Biochem., 34 (1970) 595.
Christensen, T., Acta Chem. Scand. B, 33 (1979) 763.
Castro, B., Dormoy, J.R., Evin, G. and Selve, C., Tetrahedron Lett., (1975) 1219.
King, D.S., Fields, C.G. and Fields, G.B., Int. J. Pept. Protein Res., 36 (1990) 255.
Barrett, A.J., KembhaVI, A.A., Brown, M.A., Kirschke, H., Knight, C.G., Tamai, M. and Hanada, K., Biochem. J., 201 (1982) 189.
Marquardt, D.W.J., Soc. Ind. Appl. Math., 11 (1963) 431.
Ghose, A.K. and Crippen, G.M., J. Comput. Chem., 7 (1986) 565.
Khouri, H.E., Vernet, T., Ménard, R., Parlati, F., Laflamme, P., Tessier, D.C., Gour-Salin, B., Thomas, D.Y. and Storer, A.C., Biochemistry, 30 (1991) 8929.
Author information
Authors and Affiliations
Additional information
Abbreviations used for amino acids follow the recommendations of the IUPAC-IUB Commission of Biochemical Nomenclature [Eur. J. Biochem., 138 (1984) 9]. Amino acid symbols denote the l-configuration.
Rights and permissions
About this article
Cite this article
García-Echeverría, C., Rich, D.H. Kinetic studies of papain: Effect of P3′ substituents and donor/acceptor pairs of intramolecularly quenched fluorogenic substrates. Lett Pept Sci 2, 77–82 (1995). https://doi.org/10.1007/BF00128501
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00128501