Abstract
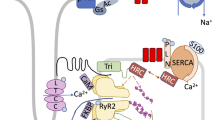
The link between cardiac contractile dysfunction in patients with end-stage heart failure and aberrant myocardial intracellular calcium handling is now well established. The precise intracellular protein(s) responsible for this breakdown in calcium handling is at present unclear. However, a number of distinct sarcolemmal (L-type, N-type, T-type, P-type, Q-type) and sarcoplasmic reticular (calcium release, ryanodine) calcium channels that have been defined on a biophysical, biochemical, and molecular basis lend valuable insights into possible factors that may contribute to the abnormal calcium handling in the hearts of these patients. What is now clear is that cardiac muscle contraction is a rigorously regulated event that follows the organized cycling of calcium from the sarcoplasmic reticulum (SR) into the cytosol and back into the SR, and that this cycle follows the graded entry of trigger calcium that enters the cell through the voltage-sensitive calcium channel. Furthermore, the voltage-dependent properties of potential-dependent calcium channels provide the underpinning for the vascular selectivity of the clinically available calcium channel drugs. Moreover, it has also been reported that the efficacy of these agents is augmented in pathologic (ischemic) tissue owing to the state dependence of these channels. Recently, the basis for the critical role of the SR in calcium signaling has started to emerge. The SR calcium handling proteins (SR calcium release channel/ryanodine receptor, SR Ca2+ ATPase, phospholamban, calsequestrin) play a critical role in maintaining intracellular free ionized calcium concentrations ([Ca2+ i), which therefore regulate systolic and diastolic function on a beat-to-beat basis within the cardiac cell. This rigorous control of [Ca2+]i is in part the result of the highly developed junctional regions of the cardiac SR. Elucidation of the calcium handling process in these regions and the potential damage resulting from cardiovascular disease has been greatly aided by the invaluable molecular tool, ryandine. From an expanding volume of information provided by animal models of ischemia, hypertrophy, and heart failure, it now appears that changes in cardiac voltage-sensitive calcium channels are likely to be the result of a secondary process that may not be directly linked to the onset of these cardiovascular diseases. Conversely, the regulation of ryanodine receptors has been suggested to be a mechanism initiating the decline in myocardial contractility leading to heart failure. These reports have been supported by studies demonstrating SR calcium release channel/ryanodine receptor changes in pressure overload hypertrophy and myocardial ischemia. Further support for the role of SR calcium release channels in cardiovascular disease is found in reports that couple leaking SR channels with ischemia and cardiac failure. These results suggest that changes in SR calcium handling proteins may be critically linked to cardiovascular disease. A more central question stemming from these results is exactly how these altered SR calcium handling proteins are involved with the onset and progression of cardiovascular disease. Application of transgenic technologies and animal models of chronic heart failure that parallel the human condition will provide the means necessary for unequivocally determining if the apparent adaptive changes in calcium handling are associated with the onset and progression of this syndrome.
Similar content being viewed by others
References
Ariai M, Alpert NR, MacLennan DH, Barton P, Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure: A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res 1993;72:463–469.
Bailey BA, Houser SR. Calcium transients in feline left ventricular myocytes with hypertrophy induced by slow progressive pressure-overload. J Mol Cell Cardiol 1992;24:265–273.
Bailey BA, Hefner C, Toaldo G-L, Dipla K, Houser SR. Calcium efflux via Na/Ca exchange is increased in hypertrophied rat ventricular myocytes. Circulation 1995;92:I-235.
Bazan E, Schwartz A, Gardner S, Wells JW, Sole MJ, Johnson CL. Receptors for calcium channel antagonists in cardiomyopathy. Fed Proc 1987;46:3125.
Bean BP. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity and pharmacology. J Gen Physiol 1985;86:1–30.
Bean BP. Multiple types of calcium channels in heart muscle and neurons. Modulation by drugs and neurotransmitters. Ann NY Acad Sci 1989;560:334–345.
Bean BP. Classes of calcium channels in vertebrate cells. Ann Rev Physiol 1989;51:367–384.
Beuckelmann DJ, Nabauer M, Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation 1992,85:1046–1055.
Bolger GT, Gengo PJ, Klockowski R, et al. Characterization of binding of the calcium channel antagonist, nitrendine, to guinea-pig ileal smooth muscle. J Pharmacol Exp Ther 1983;225:291–309.
Bowling N, Wyss VL, Gengo PJ, Utterback B, Kauffman RF, Hayes JS. Cardiac inotropic responses to calcium and forskolin are not altered by prolonged isoproterenol infusion. Eur J Pharmacol 1990;187:155–164.
Brillantes AM, Allen P, Takahashi T, Izumo S, Marks AR. Differences in cardiac calcium release channel (ryanodine receptor) expression in myocardium from patients with endstage heart failure caused by ischemci versus dilated cardiomyopathy. Circ Res 1992;71:18–26.
Bristow MR, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and beta adrenergic receptor density in failing human hearts. N Engl J Med 1982;307:205–211.
Bristow MR, Ginsburg R, Umans V, et al. 162–1 and 162–2 subpopulation in nonfailing and failing human ventricular myocardium: Coupling of both receptor subtypes to muscle contraction and selective 162–3 down regulation. Circ Res 1986;59:297–309.
Bristow MR, Hershberger RE, Port JD, Rasmussen R. 162–4 and 126–5 receptor mediated adenylate cyclase stimulation in nonfailing and failing human ventricular myocardium. Mol Pharmacol 1989;35:295–303.
Bristow MR, Feldman AM. Changes in the receptor-G protein-adenylyl cyclase system in heart failure from various types of heart muscle disease. Basic Res Cardiol 1992; 87(Suppl 1):15–35.
Cohn JN. The sympathetic nervous system in heart failure. J Cardiovasc Pharmacol 1989;14(Suppl 5):S57-S61.
Colucci WS, Ribeiro JP, Rocco MB, et al. Impaired chronotropic response to exercise In patients with congestive heart failure. Role of postsynaptic beta adrenergic desensitization. Circulation 1989;80:314–323.
Curtiss C, Cohn JN, Vrobel T, Francioza JA. The role of the renin-angiotensin system in the systemic vasoconstriction of chronic congestive heart failure. Circulation 1978; 58:763–770.
D'Angolo A, Luciani GB, Mazzucco A, Gallucci V, Salviati G. Contractile properties and calcium release activity of the sarcoplasmic reticulum in dialted cardiomyopathy. Circulation 1992;85:518–525.
Dixon IMC, Lee SL, Khalla NS. Nitrendipine binding in congestive heart failure due to myocardial infarction. Circ Res 1990;66:782–788.
Dolber DC, Sommer JR. Corbular sarcoplasmic reticulum of rabbit cardiac muscle. J Ultrastruct Res 1984;87:190–196.
Dzau VJ, Colucci WS, Hollenberg NK, Williams GH. Relation of the renin-angiotensin-aldosterone system to clinical state in congestive heart failure. Circulation 1981;63: 645–651.
Fabiato A, Fabiato F. Excitation-contraction coupling of isolated cardiac fibers with distrupted of closed sarcolemmas. Calcium-dependent cyclic and tonic contractions. Circ Res 1972;31:293–307.
Fawcett DW, McNutt NS. The ultrastructure of the cat myocardium. I. Ventricular papillary muscle. J Cell Biol 1969;42:1–45.
Feldman AM, Cates AE, Bristow MR, VanDop C. Altered expression of α-subunits of G proteins in failing human hearts. J Mol Cell Cardiol 1989;21:359–365.
Finkel MS, Marks ES, Patterson RE, Speir EC, Steadman KA, Keiser HR. Increased cardiac calcium channels in hamster cardiomyopathy. Am J Cardiol 1986;57:1205–1206.
Franzini-Armstrong C. Studies of the triad. I. Structure of the junction in frog twitch fibers. J Cell Biol 1970;47: 488–499.
Frey MJ, Molinoff PB. Mechanisms of downregulation of beta adrenergic receptors: Perspective on the role of beta adrenergic receptors in congestive heart failure. J Cardiovasc Pharmacol 1989;14(Suppl 5):S13-S18.
Fyhrquist F, Tikkanen I. Atrial natriuretic peptide in congestive heart failure. Am J Cardiol 1988;62:201–204.
Gengo PJ, Luchowski E, Rampe DE, Rutledge A, Triggle AM, Triggle DJ, Janis RA. Chemical and pharmacologic approaches to the definition and quantitation of calcium channels. Cold Spring Harb Sympo Quanti Biol 1983;48: 279–285.
Gengo PJ, Bowling NL, Wyss VL, Hayes JS. Effects of prolonged phenylephrine infusion on cardiac adrenoceptors and calcium channels. J Pharmacol Exp Ther 1988;244: 100–105.
Gengo PJ, Skattebol A, Moran JF, Gallant S, Hawthorn M, Triggle DJ. Regulation by chronic drug administration of neuronal and cardiac calcium channel, beta adrenoceptor and muscarinic receptor levels. Biochem Pharm 1988;37: 627–633.
Gengo PJ, Sabbah HN, Sharpe JK, Kono T, Stein PD, Steffen RP, Goldstein S. Regional cardiac calcium channel and beta adrenoceptor density changes in dogs with heart failure. FASEB J 1991;5:2963.
Gengo PJ, Sabbah HN, Steffen RP, Sharpe JK, Kono T, Stein PD, Goldstein S. Myocardial beta adrenoceptor and voltage sensitive calcium channel changes in a canine model of chronic heart failure. J Mol Cell Cardiol 1992;24: 1361–1369.
Go LO, Moschella MC, Watras J, Handa KK, Fyfe BS, Marks AR. Differential regulation of two types of intracellular calcium release channels during end-stage heart failure. J Clin Invest 1995;95:888–894.
Gopalakrishnan M, Triggle DJ, Rutledge A, Kwon YW, Bauer JA, Fung H-L. Regulation of potassium and calcium channels in experimental cardiac failure. Am J Physiol 1991;261:H1979-H1987.
Grossman W, Lorell BH. Diastolic Relaxation of the Heart: Basic Research and Current Applications for Clinical Cardiology. Boston: Martinus Nijhoff, 1988.
Gwathmey JK, Morgan JP. Altered calcium handling in experimental pressure-overload hypertrophy in the ferret. Circ Res 1985;57:836–843.
Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MC, Grossman W, Morgan JP. Abnormal intracellular calcium handling in myocardum from patients with endstage heart failure. Circ Res 1987;61:70–76.
Gwathmey JK, Hajjar RJ. Intracellular calcium related to force development in twitch contraction of mammalian myocardium. Cell Calcium 1990;11:531–538.
Gwathmey JK, Bentivegna LA, Ransil BJ, Grossman W, Morgan JP. Relationship of abnormal intracellular calcium mobilisation to myocyte hypertrophy in human ventricular myocardium. Cardiovasc Res 1993;27:199–203.
Hille B. Local anesthetics: Hydrophilic and hydorphobic pathways for the drug:receptor interaction. J Gen Physiol 1977;69:497–515.
Hodsman GP, Kohzuki M, Howes LG, Sumithran E, Tsunoda K, Johnston CI. Neurohumoral responses to chronic myocardial infarction in rats. Circulation 1988;78:376–381.
Holmberg SRM, Williams AJ. The calcium-release channel from cardiac sarcoplasmic reticulum: Function in the failing and acutely ischemic heart. Basic Res Cardiol 1992; 87(Suppl 1):255–268.
Homcy CJ, Vatner SF, Vatner DE. Beta adrenergic receptor regulation in the heart in pathophysiologic states; abnormal adrenergic responsiveness in cardiac disease. Annu Rev Physiol 1991;53:137–159.
Howlett SE, Gordon T. Calcium channels in normal and dystrophic hamster cardiac muscles. [3H]Nitrendipine binding studies. Biochem Pharmacol 1987;36:2653–2659.
Howlett SE, Rafuse VF, Gordon T. [3H]Nitrendipine binding studies in normal and cardiomyopathic hamsters: Absence of a selective increase in putative calcium channels in cardiomyopathic hearts. Cardiovas Res 1988;22:840–846.
Imagawa T, Takasago T, Shigekawa M. Cardiac ryanodine receptor is absent in type I slow skeletal muscle fibers: Immunochemical and ryanodine binding studies. J Biochem 1989;106:342–348.
Inui M, Saito A, Fleischer S. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J Biol Chem 1987;262:15637–15642.
Katz AM. Cardiomyopathy of overload: A major determinant of prognosis in congestive heart failure. N Engl J Med 1990;322:100–110.
Kihara Y, Grossman W, Morgan JP. Direct measurement of changes in intracellular calcium transients during hypoxia, ischemia, and reperfusion of the intact mammalian heart. Circ Res 1989;65:1029–1044.
Kim DH, Mkparu F, Kim CR, Caroll RF. Alteration of calcium release channel function in sarcoplasmic reticulum of pressure-overload-induced hypertrophic rat heart. J Mol Cell Cardiol 1994;26:1505–1512.
Kupper W. Interrupting the adaptive changes in congestive heart failure. Am J Cardiol 1991;67:20C-22C.
Lai FA, Anderson K, Rousseau E, Liu Q-Y, Meissner G. Evidence for a Ca2+ channel within the ryanodine receptor complex from cardiac sarcoplasmic reticulum. Biochem Biophys Res Commun 1988;151:441–449.
Lai FA, Erickson HF, Rousseau E, Li Q-Y, Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature 1988;331:315–319.
Lattanzio F. Alterations in intracellular free calcium and pH during global ischemia in the isolated perfused rat heart. Biophys J 1988;53:170.
Lee HC, Mohabir R, Smith N, Franz MR, Clusin WT. Effects of ischemia on calcium-dependent fluorescence transients in rabbit hearts containing indo 1: Correlation with monophasic action potentials and contraction. Circulation 1988;78:1047–1059.
MacKenzie AE, Korneluk RG, Zorzato F, Fujii J, Phillips M, Isles D, Wieringa B, Leblond S, Bailly J, Willard HF. The human ryanodine receptor gene: its mapping to 19 & 13.1, placement in a chromosome 19 linkage group. Am J Hum Genet 1990;46(6):1082–1089.
Marban E, Kitakaze M, Kusuoka H, Porterfield JK, Yeu KT, Chacko VP. Intracellular free calcium concentration measured with 19F NMR spectroscopy in intact ferret hearts. Proc Natl Acad Sci USA 1987;84:6005–6009.
Mayoux E, Callens F, Swynghedauw B, Charlemagne D. Adaptational process of the cardiac calcium channels to pressure overload: Biochemical and physiological properties of the dihydropyridine receptors in normal and hypertrophied rat hearts. J Cardiovasc Pharmacol 1988;12:390–396.
Mayoux E, Scamps F, Oliviero P, Vassort G, Charlemagne D. Calcium channels in normal and dystrophic rat heart (abstract 49). J Mol Cell Cardiol 1989;21:18.
Mazzanti M, DeFelice LJ. Calcium gating during cardiac action potentials. Biophys J 1990;58:1059–1065.
McCleskey EW, Fox AP, Feldman DH, Cruz LJ, Olivera BM, Tsien RW, Yoshikami D. ω-Conotoxin: Direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci USA 1987;84: 4327–4331.
Meerson FZ, Kapelko VI, Nourmatov AA. Physiological evaluation of the capacity of the diastole mechanism. Acta Cardiol 1971;26:547–567.
Meyer M, Schillinger W, Pieske B, et al. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation 1995;92:778–784.
Moore ED, Becker PL, Fogarty KE, Williams DA, Fay FS. Calcium imaging is single living cells: Theoretical and practical issues. Cell Calcium 1990;11:157–179.
Naudin V, Oliviero P, Rannou F, Sante Beuve C, Charlemagne D. The density of ryanodine receptors decreases with pressure overload-induced rat cardiac hypertrophy. FEBS Lett 1991;285:135–138.
O'Brien PJ, Moe GW, Cory CR, Mowack LM, Armstrong PW. Myocardial calcium and ATP cycling during the development of, and recovery from, pacing-induced heart failure. Circulation 1993;88:1526.
Otsu K, Willard HF, Khanna VK, Zorzato F, Green NM, MacLennan DH. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J Biol Chem 1990;265: 13472–13483.
Pessah IN, Waterhouse AL, Casida JE.The calcium-ryanodine receptor complex of skeletal and cardiac muscel. Biochem Biophys Res Commun 1985;128:449–456.
Pessah IN, Francini AO, Scales DJ, Waterhouse AL, Casida JE. Calcium-ryanodine receptor complex. J Biol Chem 1986;261:8643–8648.
Pfeffer MA, Pfeffer JM, Fishbein MV, Fletcher PJ, Spadaro J, Kloner RA, Brunwald E. Myocardial infarct size and ventricular dysfunction in rats. Circ Res 1979;44: 503–512.
Rannou F, Saintebeuve C, Oliverio P, Do E, Trouve P, Charlemange D. The effects of compensated cardiac hypertrophy on dihydropyridine and ryanodine receptors in rat, ferret and guinea pig hearts. J Mol Cell Cardiol 1995;27:1225–1234.
Rasmussen RP, Minobe W, Bristow MR. Calcium antagonist binding sites in failing and nonfailing human ventricular myocardium. Biochem Pharmacol 1990;39:691–696.
Reuter H. Ion channels in cardiac cell membranes. Ann Rev Physiol 1984;46:473–484.
Ringer S. A further contribution regarding the influence of the different constitutents of the blood on the contraction of the heart. J Physiol 1883;4:29–42.
rousseau E, Smith JS, Meissner G. Ryanodine modifies conductance and gating behaviour of single Ca2+ release channel. Am J Physiol 1987;253:C364-C368.
Saito A, Inui M, Radermacher J, Frank J, Fleisher S. Ultrastructure of the calcium release channel of the sarcoplasmic reticulum. J Cell Biol 1988;107:211–219.
Sanguinetti MC, Kass RS. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res 1984;55:336–348.
Sanguinetti MC, Kass RS. Regulation of cardiac calcium channel current and contractile activity by the dihydropyridine Bay K 8644 is voltage-dependent. J Mol Cell Cardiol 1984;16:667–670.
Schouten VJM, Vliegen HW, van der, Laarse A, Huysmans HA. Altered calcium handling at normal contractility in hypertrophied rat heart. J Mol Cell Cardiol 1990;22:987–988.
Sen L, Smith TW. T-type calcium channels are abnormal in genetically determined cardiomyopathic hamster hearts. Circ Res 1994;75:149–155.
Sen L, Cui G, Xu D-C, Philipson KD, Chang P, Drinkwater D, Laks H. Calcium transporting protein gene expression in myocardium from patients with ischemic or idiopathic dilated cardiomyopathy. Circulation 1995;92:I208.
Siri FM, Krueger J, Nordin C, Ming Z,Aronson DS. Depressed intracellular calcium transients and contractionin myocytes from hypertrophied and failing guinea pig hearts. Am J Physiol 1991;261:H514-H530.
Smith VE, Weisfeldt ML, Katz AM. Relazation and diastolic properties of the heart. In: Fozzard HA, Haber E. Jennings RB, Katz AM, Morgan HE, eds. The Heart and Circulation. New York: Raven Press, 1986:803–817.
Sommers JR, Johnson EA. Ultrastructure of cardiac muscle. In: Berne RM, ed. Handbook of Physiology, Vol. I. The Cardiovascular System. Bethesda, MD: American Physiological Society, 1979;113–186.
Takahashi T, Allen PD, Lacro RV, et al. Expression of dihydropyridine receptor (calcium channel) and calsequestrin genes in the myocardium of patients with end-stage heart failure. J Clin Invest 1992;90:927–935.
Triggle DJ. Desensitization. Trends Pharmacol Sci 1980;2: 395–398.
Tsunoda K, Hodsman GP, Sumithran E, Johnston CI. Atrial natuiuretic peptide in chronic heart failure in the rat: A correlation with ventricular dysfunction. Circ Res 1986; 59:256–261.
Vatner DE, Sato N, Kiuchi K, Shannon RP, Vatner SF. Decrease in myocardial ryanodine receptors and altered excitation-contraction coupling early in the development of heart failure. Circulation 1994;90:1423–1430.
Wagner JA, Sax FL, Weisman HF, et al. Calcium antagonist receptors in the atrial tissue of patients with hypertrophic cardiomyopathy. N Engl J Med 1989;320:755–761.
Wang J, Best PM. Inactivation of the sarcoplasmic reticulum calcium channel by protein kinase. Nature 1992;359: 739–741.
Wang JP, Needleman DH, Hamilton SL. Relationship of low affinity [3H]ryanodine binding sites to high affinity sites on the skeletal muscle calcium release channel. J Biol Chem 1993;268:20974–20982.
Watson MJ, Gengo PJ. Effects of ryanodine on calcium homeostasis in single adult rat cardiomyocytes. Biophys J 1995;68:A180.
Winegrad S. Autoradiographic studies of intracellular calcium in frog skeletal muscle. J Gen Physiol 1965;48: 455–479.
Williams JF. Evolving concepts in congestive heart failure. Mod Concepts Cardiovasc Dis 1990;59:43–48.
Witcher DR, Strifler BA, Jones LR. Cardiac specific phosphorylation site for multifunctional calcium calmodulin-dependent protein kinase is conserved in the brain ryanodine receptor. J Biol Chem 1992;267:4963–4976.
Zucchi R, Ronca-Testoni S, Yu G, Galbani P, Ronca G, Mariana M. Effect of ischemia and reperfusion on cardiac ryanodine receptors-sarcoplasmic reticulum calcium channels. Circ Res 1994;74:271–280.
Zhang JF, Randall AD, Ellinor PT, et al. Distinctive pharmacology and kinetics of cloned neuronal calcium channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology 1993;32:1075–1088.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gengo, P.J. Physiologic and emerging pathophysiologic role of cardiac calcium channels. Heart Failure Rev 1, 151–164 (1996). https://doi.org/10.1007/BF00126379
Issue Date:
DOI: https://doi.org/10.1007/BF00126379