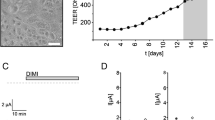
Biliary epithelial cells (BEC) were isolated from normal rat liver with high purity (> 95%) as revealed by morphological criteria as well as staining for gamma-glutamyl transferase and cytokeratin 19. During cultivation for 96 hr flattening of the cells and a loss of microvilli was apparent, while the cytokeratin 19-positive phenotype was maintained. The BEC contained a sodium-dependent as well as a sodium-independent uptake system for glutamate with high capacity. Both activities increased transiently during cultivation peaking after 72 and 48 hr, respectively. After 72 hr, apparent kinetic constants could be calculated for the sodium dependent (Km = 13.6 mM; Vmax = 388 nmoles/min/mg protein) and for the sodium-independent system. (Km = 10.8 mM; Vmax = 132 nmoles/min/mg protein). The transient increase of both transport systems was suppressed by dexamethasone. The sodium-dependence showed a threshold concentration of about 35 mM sodium. Inhibition by kainate was much less potent for BEC than for hepatocytes. These data indicate that BEC contain transport systems for glutamate different from those in hepatocytes and which may be involved in the intrahepatic reabsorbtion of glutamate from bile.
Similar content being viewed by others
Abbreviations
- BEC:
-
biliary epithelial cells
- DMEM:
-
Dulbecco's Modified Eagle's Medium
- GGT:
-
gamma-glutamyl transferase
- Dex:
-
dexamethasone
- Glu:
-
glutamate
- N-Me-AIB:
-
N-methyl-aminoisobutyrate
- Hep:
-
hepatocytes
- FBS:
-
Fetal bovine serum
References
ALPINI, G., LENZI, R., WEI-RONG, Z., LIU, M.H., SLOTT, P.A., PARONETTO, F., and TAVOLONI, N. (1989). “Isolation of a nonparenchymal liver cell fraction enriched in cells with biliary epithelial phenotypes.” Gastroenterology 97:1248–12601.
BALLATORI, N., MOSELEY, R.H., and BOYER, J.L. (1986). “Sodium gradient-dependent L-glutamate transport is localized to the canalicular domain of liver plasma membranes.” J. Biol. Chem. 261:66216–6221.
BALLATORI, N., JACOB, R., and BOYER, J.L. (1986). “Intrabiliary glutathione hydrolysis.” J. Biol. Chem. 261:7860–7865.
BURGER, H.J., GEBHARDT, R., MAYER, C., and MECKE, D. (1989). “Different capacities for amino acid transport in periportal and perivenous hepatocytes isolated by digitonin/collagenase perfusion.” Hepatology 9:22–28.
FURUKAWA, K., SHIMADA, T., ENGLAND, P., MOCHIZUKI, Y., and WILLIAMS, G.M. (1987) “Enrichment and characterization of clonigenic cells from adult rat liver and initiation of epithelial cell strains.” In Vitro 23:339–348.
GALL, J.A.M. and BHATHAL, P.S. (1985). “The isolation of intrahepatic biliary epithelial cells from normal rar livers.” Cell Biol. Int. Rep. 9:315–322.
GAZZOLA, G.C., DALL'ASTA, V., FRANCHI-GAZZOLA, R., et al. (1981). “The cluster tray method for rapid measurement of solute fluxes in adherent culture cells.” Anal. Biochem. 115:368–374.
GEBHARDT, R. (1989). “Hormonal control of amino acid transport systems in cultured periportal and perivenous hepatocytes.” In: Hepatic Transport of Organic Substances. (E. Petzinger, R.K.H. Kinne, H. Sies, eds.). Springer VerlagBerlin, Heidelberg.
GEBHARDT, R., CRUISE, J., HAUCK, K.A., LUETTEKE, N.C., NOVOTNY, A., THALER, F., and MICHALOPOULOS, G.K. (1986). “Differential effect of growth factors on growth stimulation and phenotypic stability of glutamine synthetase-positive and negative hepatocytes in primary culture.” Differentiation 333:45–55.
GEBHARDT, R. and JUNG, W. (1982). “Biliary secretion of sodium fluorescein in primary cultures of adult rate hepatocytes and its stimulation by nicotinamide.” J. Cell Sci. 56:233–244.
GEBHARDT, R. and WILLIAM, G.M. (1986). “Amino acid transport in established rat liver epithelial cell lives.” Cell. Biol. Toxicol. 2:9–20.
GEBHARDT, R. and KLEEMAN, E. (1987). “Hormonal regulation of amino acid transport system N in primary cultures of rat hepatocytes.” Eur. J. Biochem. 166:339–344.
GEBHARDT, R. and MECKE, D. (1983). “Glutamate uptake by cultured rar hepatocytes is mediated by hormonally inducible, sodium dependent transport systems.” FEBS Lett. 161:275–278.
GUZELIAN, P. and BOYER, J.L. (1974). “Glucose reabsorption from bile-evidence for a biliohepatic circulation.” J. Clin. Invest. 53:526–535.
ISHII, M., VROMAN, B., and LARUSSO, NF. (1989). “Isolation and morphologic characterization of bile duct epithelial cells form normal rat liver.” Gastroenterology 97:1236–1247.
ISHII, M., VROMAN, B., and LARUSSO, NF. (1990). “Fluid-phase endocytosis by intrahepatic bile epithelial cells isolated from normal rat liver.” J. Histochem. Cytochem. 38:515–524.
MOLL, R., FRANKE, W.W., and SCHILLER, D.L. (1982). “The catalog of human cytokeratins: pattern of expression in normal epithelia, tumors ans cultured cells.” Cell 31:11–24.
OLSON, J.R. and FUJIMOTO, J.M. (1980). “Demonstration of a D-glucose transport system in the biliary tree of the rat by use of the segmented retrograde intrabiliary injection technique.” Biochem. Pharmacol. 29:213–219.
PAROLA, M., CHEESEMAN, K.H., BIOCCA, M.E., DIANZANI, M.U., and TREVOR, F.S. (1988). “Isolation and characterization of biliary epithelial cells from normal rat liver.” J. Hepatol. 6:175–186.
RUTENBERG, A.M., KIM, H., FISCHBEIN, J.W., HANKER, J.S., WASSERKRUG, H.L., and SELIGMAN, A.M. (1969). “Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity.” J. Histochem. Cytochem. 17:517–526.
SEGLEN, P.O. (1976). “Preparation of isolated rat liver cells.” Methods Cell Biol. 13:29–83.
STERNBERGER, L.A., HARDY, P.H., CUCULIS, J.J., and MEYER, H.G. (1970). “The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase).” J. Histochem. Cytochem. 188:315–333.
STOLL, B., MCNELLY, S., BUSCHER, H.P., and HÄUSSINGER, D. (1991). “Functional hepatocyte heterogeneity in glutamate-, aspartate-and α-keto-glutarate uptake: a histoautoradiographical study.” Hepatology 13:247–253.
WILLIAMS, G.M. and GUNN, J.M. (1974). “Long-term culture of adult rat liver epithelial cells.” Exp. Cell Res 89:139–142.
YOON, Y.B., HAGEY, L.R., HOFMAN, A.F., GURANTZ, D., MICHELOTTI, E.L., and STEINBACH, J.H. (1986). “Effect of side-chain shortening on the physiologic properties of bile acids: hepatic transport and effect on biliary secretion of 23-nor-ursodeoxycholate in rodents.” Gastroenterology 90:837–852.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eisenmann-Tappe, I., Wizigmann, S. & Gebhardt, R. Glutamate uptake in primary cultures of biliary epithelial cells from normal rat liver. Cell Biol Toxicol 7, 315–325 (1991). https://doi.org/10.1007/BF00124068
Issue Date:
DOI: https://doi.org/10.1007/BF00124068