Abstract
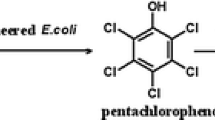
Dechlorination (para-hydroxylation) of pentachlorophenol (PCP) and tetrachloro-para-hydroquinone (TeCH) and O-methylation of TeCH were demonstrated in cell extracts of Rhodococcus chlorophenolicus PCP-I. PCP para-hydroxylating activity was membrane bound, whereas TeCH dechlorinating enzyme was soluble. The PCP para-hydroxylating enzyme was solubilized by Triton X-100 and the requirement for both FAD and NADPH was shown. The dechlorinating activities were inducible in contrast to the constitutive TeCH O-methylating activity. The PCP para-hydroxylation was inhibited by its product TeCH, by anoxic conditions, and by different inhibitors of P450. Participation of this cytochrome in the PCP hydroxylation was confirmed by the appearance of a carbon monoxide dependent peak of absorbance at 457 nm in the membrane fraction prepared from PCP degrading cells.
Similar content being viewed by others
References
Apajalahti JHA, Kärpänoja P & Salkinoja-Salonen MS (1986a) Rhodococcus chlorophenolicus sp. nov., a chlorophenol-mineralizing actinomycete. Inter. J. Syst. Bacteriol. 36: 246–251
Apajalahti JHA & Salkinoja-Salonen MS (1986b) Degradation of polychlorinated phenols by Rhodococcus chlorophenolicus. Appl. Microbiol. and Biotechn. 25: 62–67
Apajalahti JHA (1987a) Dechlorination and para-hydroxylation of polychlorinated phenols by Rhodococcus chlorophenolicus. J. Bacteriol. 169: 675–681
Apajalahti JHA (1987b) Complete dechlorination of tetrachlorohydroquinone by cell extracts of pentachlorophenol-induced Rhodococcus chlorophenenolicus. J. Bacteriol. 169: 5125–5130
Asperger O & Kleber HP (1991) Distribution and Diversity of Bacterial Cytochromes P-450. In: Rueckpaul K & Rein R (Eds) Frontiers in Biotransformation, Vol 4 (pp 1–53). Academic Verlag, Berlin
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem. 72: 248–254
Gunsalus IC & Wagner GC (1978) Bacterial P-450cam Methylene Monooxygenase Components: Cytochrome m, Putidaredoxin and Putidaredoxin Reductase. In: Fleischer S & Parker L (Eds) Methods in Enzymology (57: 166–188). Academic Press, New York
Hartmans S, van der Werf MJ & de Bont JAM (1990) Bacterial degradation of styrene involving a novel flavin adenine dinucleotide-dependent styrene monooxygenase. Appl. Environ. Microbiol. 56: 1347–1351
Häggblom MM, Apajalahti JHA & Salkinoja-Salonen MS (1988a) Hydroxylation and dechlorination of chlorinated guaiacols and syringols by Rhodococcus chlorophenolicus. Appl. Environ. Microbiol. 54: 683–687
Häggblom MM, Nohynek LJ & Salkinoja-Salonen MS (1988b) Degradation and O-methylation of chlorinated phenolic compounds by Rhodococcus and Mycobacterium strains. Appl. Environ. Microbiol. 54: 3043–3052
Knuutinen J & Kolehmainen E (1983) Synthesis and spectroscopic data of chlorinated 4-hydroxybenzaldehydes. J. Chem. Engin. Data 28: 139–141
Liu T & Chapman P (1984) Purification and properties of a plasmid-encoded 2,4-dichlorophenol hydroxylase. FEBS Lett. 2: 314–318
Müller R, Asperger O & Kleber H-P (1989) Purification of cytochrome P-450 from n-hexadecane-grown Acinetobactor calcoaceticus. Biomed. Biochim. Acta 48: 243–254
Neilson AH, Lindgren C, Hynning P-Å & Remberger M (1988) Methylation of halogenated phenols and thiophenols by cell extracts of gram-positive and gram-negative bacteria. Appl. Environ. Microbiol. 54: 524–530
Ortiz de Montellano PP, Ed (1986) Cytochrome P-450, structure, Mechanism and Biochemistry. Plenum Press, New York, 556 pp
Reineke W & Knackmuss H-J (1988) Microbial degradation of haloaromatics. Ann. Rev. Microbiol. 42: 263–287
Schenk T, Müller R, Mörsberger F, Otto MK & Lingens F (1989) Enzymatic dehalogenation of pentachlorophenol by extracts from Arthrobacter sp. strain ATCC 33790. J. Bacteriol. 171: 5487–5491
Singer VME & Finnerty WR (1988) Construction of an Escherichia coli-Rhodococcus shuttle vector and plasmid transformation in Rhodococcus sp. J. Bacteriol. 170: 638–645
Suzuki T (1978) Enzymatic methylation of pentachlorophenol and its related compounds by cell-free extracts of Mycobacterium sp. isolated from soil. J. Pestic. Sc. 3: 441–443
Straube G (1987) Phenol hydroxylase from Rhodococcus sp. P1. J. Basic Microbiol. 27: 229–232
Uotila JS, Salkinoja-Salonen MS & Apajalahti JHA (1990) Dechlorination and para-hydroxylation of pentachlorophenol by membrane-bound enzymes of R. chlorophenolicus PCP-I. Annual Meeting of American Society of Microbiology, May 13–17, Anaheim, CA, USA, Poster no. K-6
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Uotila, J.S., Salkinoja-Salonen, M.S. & Apajalahti, J.H.A. Dechlorination of pentachlorophenol by membrane bound enzymes of Rhodococcus chlorophenolicus PCP-I. Biodegradation 2, 25–31 (1991). https://doi.org/10.1007/BF00122422
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00122422