Summary
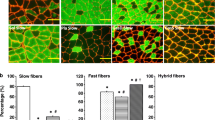
The metabolic recovery potential of muscle was studied in regenerating soleus muscles of young adult rats. Degeneration was induced by subfascial injection of a myotoxic snake venom. After regeneration for selected periods up to 2 weeks, samples of whole muscle were analysed for hexokinase (EC 2.7.1.1), phosphofructokinase (EC 2.7.1.11), lactate dehydrogenase (EC 1.1.11.27), adenylokinase (EC 2.7.4.3), creatine kinase (EC 2.7.3.2), malate dehydrogenase (EC 1.1.11.37), citrate synthase (ED 4.1.3.7) and β-hydroxyacyl CoA dehydrogenase (EC 1.1.1.35). Lactate dehydrogenase, adenylokinase, malate dehydrogenase and β-hydroxyacyl CoA dehydrogenase were also measured in individual fibres of muscle regenerating up to 4 weeks. We found that in the presence of nerve there was complete recovery of muscle metabolic capacity. However, there were differences in the rate of recovery of the activity of enzymes belonging to different energy-generating pathways. Lactate dehydrogenase, an enzyme representing glycolytic metabolism, reached normal activity immediately upon myofibre formation, only 3 days after venom injection, while oxidative enzymes required a week or more to reach normal activity levels. The delay in oxidative enzyme recovery coincided with physiological parameters of reinnervation. Therefore, to further test the role of nerve on the metabolic recovery process, muscle regeneration was studied following venom-induced degeneration coupled with denervation. In the absence of innervation, most enzymes failed to recover to normal activity levels. Lactate dehydrogenase was the only enzyme to achieve normal levels, and it did so as rapidly as in innervated-regenerating soleus muscles. The remainder of the glycotytic enzymes and the high energy phosphate enzymes recovered only partially. Oxidative enzymes showed no recovery and were severely reduced in the absence of reinnervation. Thus, it appears that enzymes of oxidative metabolism are more dependent upon innervation than enzymes of glycolytic metabolism for full expression in regenerating soleus muscle.
Similar content being viewed by others
References
ANNEX, B. H., KRAUS, B. E., LYNIS DOHM, G. & WILLIAMS, R. S. (1991) Mitochondrial biosynthesis in striated muscle: rapid induction of citrate synthase mRNA by nerve stimulation. Am. J. Physiol. 260, C266–70.
BROOKE, M. H. & KAISER, K. K. (1970) Three ‘myosin ATPase’ systems: the nature of their pH lability and sulfhydryl dependence. J. Histochem. Cytochem. 18, 670–2.
BROWN, J. M. C., HENRIKSSON, J. & SALMONS, S. (1989) Restoration of fast muscle characteristics following cessation of chronic stimulation: physiological, histochemical, metabolic changes during slow-to-fast transformation. Proc. Roy. Soc. Lond. B 235, 321–46.
BRUNETTI, A. & GOLDFINE, I. D. (1990) Role of myogenin in myoblast differentiation and its regulation by fibroblast growth factor. J. Biol. Chem. 265, 5960–3.
BUTLER-BROWNE, G. S., BUGAISKY, L. B., CUENOUD, S., SCHWARTZ, K. & WHALEN, R. G. (1982) Denervation of newborn rat muscle does not block the appearance of adult fast myosin. Nature 299, 830–3.
CARRARO, U., DALLA-LIBERA, L. & CATANI, C. (1983) Myosin light and heavy chains in regenerating muscle in the absence of nerve: transient appearance of embryonic light chain. Exp. Neurol. 79, 106–17.
CHI, M. M.-Y., HINTZ, C. S., COYLE, E. F., MARTIN, W. H., IVY, J. L., NEMETH, P. M., HOLLOSZY, J. O. & LOWRY, O. H. (1983) Effect of detraining on enzymes of energy metabolism in individual human muscle fibres. Am. J. Physiol. 244, C276–87.
CHI, M. M.-Y., HINTZ, C. S., HENRIKSSON, J., SALMONS, S., HELLEN-DAHL, R. P., PARK, J. L., NEMETH, P. M. & LOWRY, O. H. (1986) Chronic stimulation of mammalian muscle: enzyme changes in individual fibers. Am. J. Physiol. 251, C633–42.
DICKERSON, J. W. T. & WIDDOWSON, E. M. (1960) Chemical changes in skeletal muscle during development. Biochem. J. 74, 247–57.
EISENBERG, B. R. & SALMONS, S. (1981) The reorganization of subcellular structure in muscle undergoing fast-to-slow type transformation: a stereological study. Cell Tiss. Res. 220, 449–71.
FORD, D. H. & COHAN, G. (1968) Changes in weight and volumes of rat spinal cord neurons with increasing age. Acta Anat. 71, 311–19.
GORDON, T., PERRY, R., SRIHARI, T. & VRBOVA, G. (1977) Differentiation of slow and fast muscles in chickens. Cell Tiss. Res. 180, 211–22.
GRUBB, B. D. & HARRIS, J. B. (1986) The development of neuromuscular junctions on rat regenerating skeletal muscle fibres. J. Physiol. 380, 65P.
HAMM, T. M., NEMETH, P. M., SOLANKI, L., GORDON, D. A., REINKING, R. M. & STUART, D. G. (1988) Association between biochemical and physiological properties in rat single motor units. Muscle Nerve 11, 95–104.
HARRIS, J. B. (1981) Reconstruction of neuromuscular junctions in rat skeletal muscle following assault by the mytoxic neurotoxin notexin. Brit. J. Pharm. 74, 249–50P.
HARRIS, J. B. & JOHNSON, M. A. (1978) Further observations on the pathological responses of rat skeletal muscle to toxins isolated from the venom of the Australian tiger snake, Notechis scutatus scutatus. Clin. Exp. Pharm. Physiol. 5, 587–600.
HARRIS, J. B., JOHNSON, M. A. & KARLSSON, E. (1975) Pathological responses of rat skeletal muscle to a single subcutaneous injection of a toxin isolated from the venom of the Australian tiger snake, Notechis scutatus scutatus. Clin. Exp. Pharm. Physiol. 2, 383–404.
HENRIKSSON, J., CHI, M. M.-Y., HINTZ, C. S., YOUNG, D. A., KAISER, K. K., SALMONS, S. & LOWRY, O. H. (1986) Chronic stimulation of mammalian muscle: changes in enzymes of six metabolic pathways. Am. J. Physiol. 251, C614–33.
HINTZ, C. S., LOWRY, C. V., KAISER, K. K., McKEE, D. & LOWRY, O. H. (1980) Enzyme levels in individual rat muscle fibres. Am. J. Physiol. 239, C58–65.
JOLESZ, F. & SRETER, F. A. (1981) Development, innervation and activity-induced changes in skeletal muscle. Ann. Rev. Physiol. 43, 531–2.
KELLY, A. M., ROSSER, B. W. C., HOFFMAN, R., PANETTIERI, R. A., SCHIAFFINO, S., RUBINSTEIN, N. A. & NEMETH, P. M. (1991) Metabolic and contractile protein expression in developing rat diaphragm muscle. J. Neurosci. 11, 1231–41.
LOWRY, C. V., KIMMEY, J. S., FELDER, S., CHI, M. M.-Y., KAISER, K. K., PASSONNEAU, J. V., NEMETH, P. M., KIRK, K. A. & LOWRY, O. H. (1978) Enzyme patterns in single human muscle fibers. J. Biol. Chem. 253, 8269–77.
MAURO, A. (1979) Muscle regeneration. New York: Raven Press.
MANCHESTER, J. K., CHI, M. M.-Y., NORRIS, B., FERRIER, B., KRASNOV, I., NEMETH, P. M., McDOUGAL, D. B. & LOWRY, O. H. (1990) Effects of microgravity on metabolic enzymes of individual muscle fibers. FASEB J. 4, 55–63.
MARECHAL, G., SCHWARTZ, K., BECKERS-BLEUKX, G. & GHINS, E. (1984) Isozymes of myosin in growing and regenerating rat muscles. Eur. J. Biochem. 138, 421–8.
NAVARRETE, R. & VRBOVA, G. (1983) Changes of activity patterns is slow and fast muscles during postnatal development. Dev. Brain Res. 8, 11–19.
NENETH, P. M., NORRIS, B. J., SOLANKI, L. & KELLY, A. M. (1989) Metabolic specialization in fast and slow muscle fibers of the developing rat. J. Neurosci. 9, 2336–43.
NEMETH, P. M., SOLANKI, L., GORDON, D. A., HAMM, T. M., REINKING, R. M. & STUART, D. G. (1986) Uniformity of metabolic enzymes within individual motor units. J. Neurosci. 6, 892–8.
NYSTROM, B. (1968) Fibre diameter increases in nerves to ‘slow-red’ and ‘fast white’ cat muscles during postnatal development. Acta Neurol. Scand. 44, 265–94.
PASSONNEAU, J. V. & LOWRY, O. H. (1993) Enzymatic Analysis: A practical guide. Iotowa, N.J.: Humana Press.
PETTE, D., RAMIREZ, B. U., MULLER, W., SIMON, R., EXNER, G. E. & HILDEBRAND, R. (1975) Influence of intermittent long-term stimulation on contractile histochemical and metabolic properties of fiber populations in fast and slow rabbit muscles. Pflügers Arch. 361, 1–7.
PETTE, D., SMITH, M. E., STAUDTE, H. W. & VRBOVA, G. (1973) Effects of long-term stimulation on some contractile and metabolic characteristics of fast and slow rabbit muscles. Pflügers Arch. 338, 257–72.
REICHMANN, H., HOPPELER, H., MATHIEU-COSTELLO, O., VonBERGEN, F. & PETTE, D. (1985) Biochemical and ultrastructural changes of skeletal muscle mitochondria after chronic electrical stimulation in rabbits. Pflügers Arch 404, 1–9.
SALMONS, S. & HENRIKSSON, J. (1981) The adaptive response of skeletal muscle to increased use.Muscle Nerve 4, 94–105.
SATO, M., MIZUNO, N. & KONISHI, A. (1977) Postnatal differentiation of cell body volumes of spinal motoneurones innervating slow-twitch and fast-twitch muscles. J. Comp. Neurol. 175, 27–36.
SCHMALBRUCH, H. (1985) Skeletal muscle. In Handbook of microscopic anatomy, vol. 2, part 6. (edited by, Oksche, A. & Vollerth, L.), pp. I-400. Berlin: Springer Verlag.
SESODIA, S. & CULLEN, M. J. (1991) The effect of denervation on the morphology of regenerating rat soleus muscle. Acta Neuroph. 82, 21–32.
SEEDORF, U., LIBERER, E., KIRSCHBAUM, B. J. & PETTE, D. (1986) Neural control of gene expression in skeletal muscle. Effects of chronic stimulation on lactate dehydrogenase isoenzymes and citrate synthase. Biochem. J. 239, 115–20.
SIMONEAU, J. A. & PETTE, D. (1988) Species-specific effects of chronic nerve stimulation upon tibialis anterior muscle in mouse, rat, guinea pig, and rabbit. Pflügers Arch. 412, 86–92.
SOHAL, G. S. & HOLT, R. K. (1980). Role of innervation in the embryonic development of skeletal muscle. Cell Tiss. Res. 210, 383–93.
WEBER, F. E. & PETTE, D. (1988) contractile activity enhances the synthesis of hexokinase II in rat skeletal muscle. FEBS Lett. 238, 71–3.
WESTERGA, J. & GRAMSBERGEN, A. (1993) Changes in the electromyogram of two major hindlimb muscles during locomotor development in the rat. Exp. Brain Res. 92, 479–88.
WEYDERT, A., DAUBAS, P., CARAVATTI, M., MINTY, A., BUGAISKY, G., COHEN, A., ROBERT, B. & BUCKINGHAM, M. (1983) Sequential accumulation of mRNAs encoding different myosin heavy chain isoforms during skeletal muscle development in vivo detected with a recombinant plasmid identified as coding for an adult fast myosin heavy chain from mouse skeletal muscle. J. Biol. Chem. 248, 13876–81.
WHALEN, R. G., HARRIS, J. B., BUTTER-BROWNE, G. S. & SESODIA, S. (1990) Expression of myosin isoforms during Notexin-induced regeneration of rat soleus muscles. Dev. Biol. 141, 24–40.
WHALEN, R. G., SELL, S. M., BUTLER-BROWNE, G. S., SCHWARTZ, K., VOUVERET, P. & PINSET-HARSTROM, J. (1981) Three myosin heavy chain isozymes appear sequentially in rat muscle development. Nature 292, 805–9.
WILLS, K. N. & MANSOUR, T. E. (1990) Changes in phosphofructokinase isozymes during development of myoblasts to myotubes. Arch. Biochem. Biophys. 278, 81–7.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sesodia, S., Choksi, R.M. & Nemeth, P.M. Nerve-dependent recovery of metabolic pathways in regenerating soleus muscles. J Muscle Res Cell Motil 15, 573–581 (1994). https://doi.org/10.1007/BF00121163
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00121163