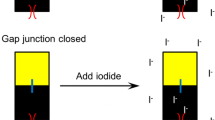
Inhibition of gap junction-mediated cell-cell communication might be a mechanism for several types of cellular dysfunctions, including tumor promotion. Although many different assays have been designed to measure gap junction-mediated intercellular communication, we applied a new technique, termed Fluorescence Redistribution After Photobleaching (“FRAP”), to assess the ability of a known tumor promoter, 2,2′, 4,4′, 5,5′-hexabromobiphenyl (245-HBB), to inhibit cell-cell communication in a concentration-dependent manner. WB-F344 (rat epithelial) cells were plated at low density, exposed to noncytotoxic concentrations of 1, 5, or 20 µg 245-HBB/ml medium, and stained with 6-carboxyfluorescein diacetate. Single cells in pairs or clusters of touching cells in each exposure group were examined with FRAP. The results revealed an inverse correlation between the degree offluorescence redistribution in photobleached cells and the concentration of 245-HBB. Therefore, FRAP appears to be a sensitive and rapid technique for determining complete or partial inhibition of chemically induced intercellular communication in vitro. These results also provide further evidence for the ability of 245-HBB to inhibit gap junction-mediated cell-cell communication in a concentration-dependent manner.
Similar content being viewed by others
Abbreviations
- 6-CFDA:
-
6-carboxyfluorescein diacetate
- FRAP:
-
fluorescence redistribution after photobleaching
- 245-HBB:
-
2,2′, 4,4′, 5,5′-hexabromobiphenyl
References
CHEN, T.R. (1977). In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp. Cell Res. 104:255–262.
DEMELLO, W.C. (1982). Cell-cell communication in heart and other tissues. Prog. Biophys. Mol. Biol. 39:147–182.
EL-FOULY, M.H., TROSKO, J.E. and CHANG, C.C. (1987). Scrape-loading and dye transfer: A rapid and simple technique to study gap junctional intercellular communication. Exp. Cell Res. 168:422–430.
ENOMOTO, T., SASAKI, Y., SHIBA, Y., KANNO, Y. and YAMASAKI, H. (1981). Inhibition of the formation of electrical cell coupling of FL cells by tumor promoters. Gann 72:631–634.
EVANS, M.G. EL-FOULY, M.H., TROSKO, J.E. and SLEIGHT, S.D. (1988). Anchored cell analysis and sorting coupled with the scrape-loading/dye transfer technique to quantify inhibition of gap junctional intercellular communication in WB-F344 cells by 2,2′, 4,4′, 5,5′-hexabromobiphenyl. J. Toxicol. Environ. Health, in press.
FITZGERALD, D.J. and MURRAY, A.W. (1980). Inhibition of intercellular communication by tumor-promoting phorbol esters. Cancer Res. 40:2935–2937.
JOHNSON, K.R., PANTER, S.S. and JOHNSON, R.G. (1985). Phosphorylation of lens membranes with a cyclic AMP-dependent protein kinase purified from the bovine lens. Biochim. Biophys. Acta 844:367–376.
JONE, C., TROSKO, J.E. and CHANG, C.C. (1987). Characterization of a rat epithelial cell line to detect inhibitors of metabolic cooperation. Cell. Devel. Biol. 23:214–220.
KAVANAGH, T.J., RUBINSTEIN, C., LIU, P.I., CHANG, C.C., TROSKO, J.E. and SLEIGHT, S.D. (1985). Failure to induce mutations in Chinese hamster V-79 cells and WB rat liver cells by the polybrominated biphenyls Firemaster BP-6, 2,2′, 4,4′, 5,5′-hexabromobiphenyl, 3,3′, 4,4′, 5,5′-hexabromobiphenyl, and 3,3′, 4,4′-tetrabromobiphenyl. Toxicol. Appl. Pharmacol. 79:91–98.
KAVANAGH, T.J., CHANG, C.C. and TROSKO, J.E. (1987). Effect of various polybrominated biphenyls on cell-cell communication in cultured human teratocarcinoma cells. Fund. Appl. Toxicol. 8:127–131.
LAMPE, P.D., BUZZI, M.D., NELSESTUEN, G.L. and JOHNSON, R.D. (1986). Phosphorylation of lens intrinsic membrane proteins by protein kinase C. Eur. J. Biochem. 156:351–357.
LARSEN, W.J. (1983). Biological implications of gap junction structure, distribution, and composition: A review. Tissue Cell 15:645–671.
LOEWENSTEIN, W.R. (1979). Junctional intercellular communication and the control of growth. Biochim. Biophys. Acta 560:1–65.
MURRAY, A.W. and FITZGERALD, D.T. (1979). Tumor promoters inhibit metabolic cooperation in cocultures of epidermal and 3T3 cells. Biochem. Biophys. Res. Commun. 91:395–401.
NEWBOLD, R.F. and AMOS, J. (1981). Inhibition of metabolic cooperation between mammalian cells in culture by tumor promoters. Carcinogenesis 2:243–249.
ROSE, B. and LOEWENSTEIN, W.R. (1975). Permeability of cell junctions depends on local cytoplasmic calcium activity. Nature 254:250–252.
SAEZ, J.E., SPRAY, D.C., NAIRN, A.C., HERTZBERG, E., GREENGARD, P. and BENNETT, M.V.L. (1986). cAMP increases junctional conductance and stimulates phosphorylation of the 27-KDa principle gap junction polypeptide. Proc. Natl. Acad. Sci. USA 83:2473–2477.
SAEZ, J.C., BENNETT, M.V.L. and SPRAY, D.C. (1987). Carbon tetrachloride at hepatotoxic levels blocks reversibly gap junctions between hepatocytes. Science 236:967–969.
SOCOLAR, S.J. and LOEWENSTEIN, W.R. (1978). Methods for studying transmission through permeable cell-to-cell junctions. In: Methods in Membrane Biology (E. Korn, ed.), Vol. 10, pp. 123–179. Plenum Press, New York.
SUBAK-SHARPE, H., BURK, R.R. and PITTS, J.D. (1969). Metabolic cooperation between biochemically marked mammalian cells in culture. J. Cell Sci. 4:353–367.
TAKEDA, A., HASHIMOTO, E., YAMAMURA, H. and SHIMAZU, T. (1987). Phosphorylation of liver gap junction protein by protein kinase C. FEBS Lett. 210:169–172.
TENNANT, R.W., MARGOLIN, B.H., SHELBY, M.D., ZEIGER, E., HASEMAN, J.K., SPALDING, J., CASPARY, W., RESNICK, M., STASIEWICZ, S., ANDERSON, B. and MINOR, R. (1987). Prediction of chemical carcinogenicity in rodents from in vitro genetic toxicity assays. Science 236:933–941.
TROSKO, J.E., YOTTI, L.P., WARREN, S.T., TSUSHIMOTO, G. and CHANG, C.C. (1982). Inhibition of cell-cell communication by tumor promoters. Carcinogenesis 3:181–186.
TROSKO, J.E., ONE, C. and CHANG, C.C. (1983a). The role of tumor promoters on phenotypic alterations affecting intercellular communication and tumorigenesis. In: Cellular Systems for Toxicity Testing (G.M. Williams, V.C. Dunkel, and V.A. Ray, eds.) pp 316–327, New York Academy of Sciences, New York.
TROSKO, J.E., CHANG, C.C. and METCALF, A. (1983b). Mechanisms of tumor promotion: Potential role of intercellular communication. Cancer Invest. 1:511–526.
TROSKO, J.E., JONE, C. and CHANG, C.C. (1987). Inhibition of gap junctional-mediated intercellular communication, in vitro, by aldrin, dieldrin and toxaphene: A possible cellular mechanism for their tumor promoting and neurotoxic effects. Molec. Toxicol. 1:83–93.
TSAO, M.S., SMITH, J.D., NELSON, K.G. and GRISHAM, J.W. (1984). A diploid epithelial cell line from normal adult rat liver with phenotypic properties of “oval” cells. Exp. Cell Res. 154:38–52.
TSAO, M.S., GRISHAM, J.W., NELSON, K.G. and SMITH, J.D. (1985). Phenotypic and karyotypic changes induced in cultured rat hepatic epithelial cells that express the “oval” cell phenotype by exposure to N-methyl-N′-nitro-N-nitrosoguanidine. Am. J. Pathol. 118:306–315.
TSUSHIMOTO, G., ASANO, S., TROSKO, J.E. and CHANG, C.C. (1983a). Inhibition of intercellular communication by various congeners of polybrominated biphenyl and polychlorinated biphenyl. In: PCBs: Human and Environmental Hazards (F.M. D'Itri and M.A. Kamrin, eds.) pp. 241–252, Butterworth Publishers, Woburn, Massachusetts.
TSUSHIMOTO, G., TROSKO, J.E., CHANG, C.C. and AUST, S.D. (1983b). Inhibition of metabolic cooperation in Chinese hamster V79 cells in culture by various polybrominated biphenyl (PBB) congeners. Carcinogenesis 4:181–185.
TURIN, L. and WARNER, R.E. (1978). Carbon dioxide reversibly abolishes ionic communication between cells of early amphibian embryo. Nature 220:56–57.
WADE, M.H., TROSKO, J.E. and SCHINDLER, M. (1986). A fluorescence photobleaching assay of gap junction-mediated communication between human cells. Science 232:525–528.
WIENER, E.C. and LOEWENSTEIN, W.R. (1983). Correction of cell-cell communication defect by introduction of a protein kinase into mutant cells. Nature 305:433.
WILLIAMS, G.M. (1981). Liver carcinogenesis: The role for some chemicals of an epigenetic mechanism of liver-tumor promotion involving modification of the cell membrane. Food Cosmet. Toxicol. 5:577–583.
WILLIAMS, G.M., TONG, C. and TELANG, S. (1984). Polybrominated biphenyls are non-genotoxic and produce an epigenetic membrane effect in cultured liver cells. Environ. Res. 34:310–320.
YAMASAKI, H., ENOMOTO, T., MARTEL, N., SHIBA, Y. and KANNO, Y. (1983). Tumor promoter-mediated reversible inhibition of cell-cell communication (electrical coupling). Exp. Cell Res. 146:297–308.
YOTTI, L.P., CHANG, C.C. and TROSKO, J.E. (1979). Elimination of metabolic cooperation in Chinese hamster cells by a tumor promoter. Science 206:1089–1091.
Author information
Authors and Affiliations
Additional information
Michigan Agricultural Experiment Station journal article No. 12531.
Rights and permissions
About this article
Cite this article
Evans, M.G., Trosko, J.E. Concentration/response effect of 2,2′, 4,4′, 5,5′-hexabromobiphenyl on cell-cell communication in vitro: assessment by fluorescence redistribution after photobleaching (“FRAP”). Cell Biol Toxicol 4, 163–171 (1988). https://doi.org/10.1007/BF00119243
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00119243