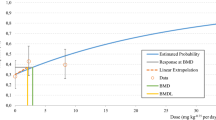
Chemical risk assessment is a complex process that requires integration of various biological data from test species, ultimately producing a prediction of the expected outcome of anticipated human exposure. There are two aspects of this process in which pharmacokinetic (PK) modeling can play an important role: in dosimetry, the process of estimating target tissue dose in the test species, and in extrapolation, the process of generalizing beyond the test species to predict human target tissue dose for various ambient exposure conditions. Mechanistic information on the cancer process is crucial in selecting the appropriate measure of target tissue dose: i.e., is it tissue exposure to parent chemical, tissue exposure to stable or reactive metabolite(s), occupancy of critical cellular receptors by parent or metabolite, or some measure of cytotoxicity with concommitant reparative hyperplasia? (This is not intended, by the way, to be an exhaustive list of the potential measures of tissue dose associated with cancer induction.) With a presumed carcinogenic mechanism and its appropriate measure of tissue dose in mind, a pharmacokinetic model can then be developed to quantitate this measure of target tissue dose for various exposure conditions. Physiologically based pharmacokinetic (PB-PK) modeling is the preferred modeling strategy since it is more readily amenable to the interspecies extrapolation necessary to calculate human tissue dose. This essay focuses on the issues of what constitutes an appropriate measure of tissue dose and of how PB-PK models can be developed to estimate tissue dose for chemicals which cause cancer by differing mechanisms. It outlines preliminary attempts to include information on cytotoxicity into a quantitative risk assessment process. Quantitative, extrapolable cytotoxicity models are necessary to conduct biologically valid risk assessments for those chemicals whose primary effect is overt cellular toxicity instead of direct chemical interaction with cellular DNA. Rational, comprehensive risk assessments will only be possible with the advent of descriptions which combine information on both pharmacokinetics and pharmacodynamics into a single integrated model.
Similar content being viewed by others
References
ANDERSEN, M.E. (1981). Saturable metabolism and its relationship to toxicity. Crit. Rev. Toxicol. 9:105–150.
ANDERSEN, M.E., CLEWELL, H.J., III, GARGAS, M.M., SMITH, F.A., and REITZ, R.H. (1987). Physiologically-based pharmacokinetics and the risk assessment process for methylene chloride. Toxicol. Appl. Pharmacol. 87:185–205.
CLEWELL, H.J., III and ANDERSEN, M.E. (1985). Risk assessment extrapolations and physiological modeling. Toxicol. Ind. Health. 1:111–131.
CONOLLY, R.B., REITZ, R.H. and ANDERSEN, M.E. (1987). Mutation accumulation: a biologically-based mathematical model of chronic cytotoxicant exposure. Drinking water and health: pharmacokinetics in risk assessment, Vol. 8. National Academy Press, Washington, D.C.
GERLOWSKI, L.E. and JAIN, R.K. (1983). Physiologically based pharmacokinetic modeling: principles and applications. J. Pharm. Sci. 72:1103–1127.
MOOLGAVKAR, S.H. This symposium.
MOOLGAVKAR, S.H. and KUNDSON, A.G., Jr. (1981). Mutation and cancer. A model for human carcinogenesis. J. Natl. Cancer Inst. 66:1037–1052.
MOOLGAVKAR, S.H. (1986). Carcinogenesis modeling: from molecular biology to epidemiology.7:151–169.
NATIONAL RESEARCH COUNCIL. (1986). Dose-route extrapolations: using inhalation toxicity data to set drinking water limits. pp. 168–225 in Drinking Water and Health, Vol. 6. Washington, D.C.: National Academy Press.
REITZ, R.H. (1987). The role of cytotoxicity in the carcinogenic process. In: Nongenotoxic Mechanisms in Carcinogenesis. Banbury Report 25, Cold Spring Harbor, N.Y., Cold Spring Harbor Laboratory.
Author information
Authors and Affiliations
Additional information
Paper presented at: Cancer Risk Assessment Symposium, Annual Meeting Society of Toxicology, Washington, D.C., February, 1987.
Rights and permissions
About this article
Cite this article
Andersen, M.E. Tissue dosimetry, physiologically-based pharmacokinetic modeling, and cancer risk assessment. Cell Biol Toxicol 5, 405–415 (1989). https://doi.org/10.1007/BF00118411
Issue Date:
DOI: https://doi.org/10.1007/BF00118411