Abstract
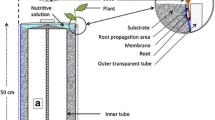
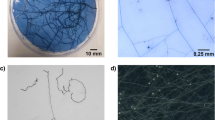
The feasibility of using a whole plant microculture system coupled with image analysis to observe and quantify elusive root growth phenomena was demonstrated. Subtle differences in root initiation and growth rate for maple microcuttings inserted into three distinct rooting media were recurrently registered over the span of the rooting phase in terms of root length, number, and weighted density (equivalent to fresh weight) without disturbing the rooting environment. This method provided a non-intrusive time-course assay for take-all disease symptom expression on wheat lines (degree and rate of root lesion development), which paralleled the relative resistance rated in field plots. Although agar-solidified media had a relatively high light transmission (nearly 84% through 6.6 cm medium depth), roots could only be clearly perceived through a thickness of about 2 cm, and were often completely obscured when embedded to a depth greater than 5 cm. However, there was no detectable loss of definition for roots observed through Gelrite-solidified media (non-diffuse light transmission of 97%) up to a depth of 6.6 cm.
Similar content being viewed by others
Abbreviations
- BA:
-
benzyl adenine
- FW:
-
fresh weight
- IBA:
-
indole-3-butyric acid
- TDZ:
-
thidiazuron
- WD:
-
weighted density
References
Arkin GF (1981) Root zone modification: systems considerations and constraints. In: Arkin GF & Taylor HM (Eds) Modifying the Root Environment to Reduce Crop Stress (p 393–403). Am. Soc. Agric. Eng. Monograph 4, St Joseph, Michigan
Bentz SE, Stimart DP & McIntosh MS (1985) Root and shoot growth patterns of newly rooted woody plants. J. Am. Soc. Hortic. Sci. 110:308–313
Holden J (1976) Infection of wheat seminal roots by varieties of Phialophora radicola and Gaeumannomyces graminis. Soil. Biol. Biochem. 8:109–119
Ivanicka J (1987) In vitro micropropagation of Mulberry, Morus nigra L.. Sci. Hortic. 32:33–39
Lakso AN, Reisch BI, Mortensen J & Roberts MH (1986) Carbon dioxide enrichment for stimulation of growth of in vitro-propagated grapevines after transfer from culture. J. Am. Soc. Hortic. Sci. 111:634–638
Lee N, Wetzstein HY & Sommer HE (1986) The effect of agar vs. liquid medium on rooting in tissue-cultured sweetgum. HortScience 21:317–318
Meyer MJ, Smith MAL & Knight SL (1989) Salinity effects on St. Augustinegrass: A novel system to quantify stress response. J. Plant Nutr. 12:893–908
Murakami T & Yoneyama T (1988) Comparison of root length of two rice (Oryza sativa L.) varieties by using an image analyzer. Plant and Soil 105: 287–289
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473–497
Pearson RW (1974) Significance of rooting pattern to crop production and some problems of root research. In: Carson EW (Ed) The Plant Root and Its Environment (pp 240–270). Univ Press of Virginia, Charlottesville, VA
Penrose L (1985) Evidence for resistance in wheat cultivars grown in sand culture to the take-all pathogen, Gaeumannomyces graminis var. tritici. Ann. Appl. Biol. 107: 105–108
Pieper M & Smith MAL (1988) A model whole plant microculture system for Kentucky bluegrass (Poa pratensis L.) Crop Sci. 28:611–614
Smith MAL & Neely D (1981) Screening woody ornamental cuttings for propagation diseases. Plant Dis. 65: 893–895
Smith MAL, Spomer LA, Meyer MJ & McClelland MT (1989) Non-invasive image analysis evaluation of growth during plant micropropagation. Plant Cell, Tiss. Org. Cult. 19:91–102
Wallwork H (1989) Screening for resistance to take-all in wheat, triticale and wheat-triticale hybrid lines. Euphytica 40:103–109
White PR (1943) A Handbook of Plant Tissue Culture. The Jacques Cattell Press, Lancaster, Pennsylvania
Wulster GJ (1985) A nondestructive method for measuring root system surface area. HortScience 20:1058–1060
Young E & Werner J (1984) A nondestructive method for measuring shoot and root fresh weights. HortScience 19:554–555
Zimmerman RH (1984) Rooting apple cultivars in vitro: Interactions among light, temperature, phloroglucinol and auxin. Plant Cell, Tissue Org. Cult. 5:301–311
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Smith, M.A.L., Spomer, L.A. & McClelland, M.T. Direct analysis of root zone data in a microculture system. Plant Cell Tiss Organ Cult 23, 21–26 (1990). https://doi.org/10.1007/BF00116085
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00116085