Abstract
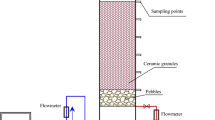
As humic substances left in treated water tend to form trihalomethans during chlorination, their removal in water treatment processes is a significant concern for drinking water supplies. One of the removal technologies, the biofilm reactor is studied for the microbial decomposition of aquatic fulvic acid (AFA). The AFA is characterized by elemental analysis, UV-Vis, 13C-NMR, and IR spectroscopic methods. The spectroscopic and elemental investigation was capable of characterizing the microbial decomposition of AFA. Biologically treated fulvic acid was in a more oxidized state; its spectra displayed a higher degree of condensation of aromatic constituents than influent fulvic acid. Microbial degradation of AFA was more active in the low molecular weight fractions and intensively occurred in the aliphatic fraction.
Similar content being viewed by others
Abbreviations
- Aλ :
-
the absorbance at wavelength
- αλ :
-
specific absorptivities
- AFAs:
-
aquatic fulvic acids
- AHS:
-
aquatic humic substances
- COD:
-
chemical oxygen demand
- Da:
-
dalton
- DO:
-
dissolved oxygen
- E4/E6ratio:
-
a ratio between absorbance at 465 and 665 nm
- FA:
-
fulvic acid
- IR:
-
infrared
- NMR:
-
nuclear magnetic resonance
- TOC:
-
total organic carbon
- UV-Vis:
-
ultraviolet-visible
References
Aiken GR (1984) Evaluation of ultrafiltration for determining molecular weight of fulvic acid. Environ. Sci. Technol. 18: 978–981
Aiken GR, McKnight DM, Wershaw RL & MacCarthy P (1985) An introduction to humic substances in soil, sediment and water. In: Aiken GR, McKnight DM & Wershaw RL Eds) Humic Substances in Soil, Sediment and Water (pp 1–12). Wiley & Sons, New York
Breemen ANvan, Nieuwstad ThJ & Meent-Olieman GCvan der (1979) The fate of fulvic acids during water treatment. Wat. Sci. Technol. 13: 771
Chen Y, Senesi N & Schnitzer M (1977) Information provided on humic substances by E4/E6 ratios. Soil Sci. Soc. Am. J. 41: 352–358
Edzwald JK, Becker WC & Tambini SJ (1987) Organics, polymer, and performance in direct filtration. J. Env. Eng. 113: 167–185
Flaig E, Beutelspacher H & Rietz E (1975) Chemical composition and physical properties of humic substances. In: Gieseking JE (Ed) Soil Components: Organic Components, Vol. 1 (pp 1–212). Springer, New York
Haan Hde (1974) Effect of a fulvic acid fraction on the growth of a Pseudomonas from Tjeukemeer (The Netherlands). Freshwater Biol. 4: 301–310
Haan Hde (1977) Effect of benzoate on microbial decomposition of fulvic acids in Tjeukemeer (The Netherlands). Limnol. Oceanogr. 22: 38–44
Haan Hde & Boer Tde (1978) A study of the possible interactions between fulvid acids, amino acids and carbohydrates from Tjeukemeer, based on gel filtration at pH 7.0. Water Res. 12: 1035–1040
Hatcher PG, Rowan R & Mattingly MA (1980) 1H and 13C-NMR of marine humic acids. Organic Geochem. 2: 77–85
Heizlar J, Szpakowska B & Wershaw RL (1994) Comparison of humic substances isolated from peatbog water by sorption on DEAE-Cellulose and Amberlite XAD-2. Wat. Res. 28:, 1961–1970
Inbar Y, Chen Y & Hadar Y (1990) Humic substances formed during the composting of organic matter. Soil Sci. Soc. Am J. 54: 1316–1323
Nash KL & Choppin GR (1980) Interaction of humic and fulvic acids with Th(IV). J. Inorg. Nucl. Chem. 42: 1045–1050
Preston CM & Schnitzer M (1987) 13C-NMR of humic substances: pH and solvent effects. J. Soil Sci. 38: 667–678
Rebhun M & Lurie M (1993) Control of organic matter by coagulation and floc separation. Wat. Sci. Tech. 27: 1–20
Rittmann BE & Snoeyink VL (1984) Achieving biologically stable drinking water. JAWWA 76: 106
Rittmann BE (1985) Biological processes and organic micropollutants in treatment processes. Sci. Total Environ. 47: 99
Rook JJ (1977) Chlorination reactions of fulvic acids in natural waters. Environ. Sci. Technol. 11: 168–172
Shapiro J (1957) Chemical and biological studies on the yellow organic acids of lake water. Limnol. Oceanogr. 2: 161–179
Shin HS, Moon HC, Yang HB & Yun SS (1994) Spectroscopic investigation of humic materials. Bull. Korean Chem. Soc. 15: 777–781
Stabel HH, Moaledj K & Overbeck J (1979) On the degradation of dissolved organic molecules from Plussee by oligocarbophilic bacteria. Arch. Hydrobiol. Beih. Ergebn. Limnol. 12: 95–104
Stanier RY & Ornston LN (1973) The β-ketoadipate pathway. Adv. Microbiol. Physiol. 9: 89–151
Steelink C (1985) Implication of elemental characteristics of humic substances. In: Aiken GR, McKnight DM & Wershaw RL (Eds) Humic Substances in Soil, Sediment and Water (pp 457–476). Wiley & Sons, New York
Stevenson FJ & Goh KM (1972) Infrared spectra of humic and fulvic acids and their methylated derivatives: evidence for nonspecificity of analytical methods for oxygen containing functional groups. Soil Sci. 113: 334–345
Stevenson FJ (1982) Humus Chemistry: Genesis, Composition Reactions. John Wiley & Sons, New York
Thurman EM & Malcolm RL (1983) Structural study of humic substances: new approaches and methods. In: Christman RF & Gjessing ET (Eds) Aquatic and Terrestrial Humic Materials (pp 1–23). Ann Arbor Science, Ann Arbor MI
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shin, HS., Lim, KH. Spectroscopic and elemental investigation of microbial decomposition of aquatic fulvic acid in biological process of drinking water treatment. Biodegradation 7, 287–295 (1996). https://doi.org/10.1007/BF00115742
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00115742