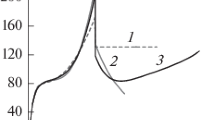
The specific heats of UAs and the isostructural nonmagnetic homolog ThAs have been measured in the temperature range 5–300 K. While the latter compound displays a regular smooth curve C p (T), UAs shows two sharp anomalies. The first anomaly, around 64 K, may be ascribed to the magnetic transition from type IA to type I antiferromagnetic structure; the second anomaly, at 122.8 K, corresponds to the Néel temperature. An analysis of the experimental curve C p (T) for UAs has been carried out by several different methods to get the magnetic contribution to the specific heat with the best possible accuracy. The resulting magnetic entropy depends on the method and its maximum value at 250 K is 0.8 R ln 4, assuming a high-temperature value of the electronic heat capacity coefficient 〈γ〉 − 33 mJ/K2 mole. No anomaly at 41 K was observed whatever thermal treatment was used to prepare the UAs samples.
Similar content being viewed by others
References
J. F. Counsell, R. M. Dell, and J. F. Martin, Trans. Faraday Soc. 62, 1736 (1966).
E. F. Westrum, Jr. and C. M. Barber, J. Chem. Phys. 45, 635 (1966).
J. F. Counsell, R. M. Dell, A. R. Junkison, and J. F. Martin, Trans. Faraday Soc. 63, 72 (1967).
H. Yokokawa, Y. Takahashi, and T. Mukaibo, in Thermodynamics of Nuclear Materials (IAEA, Vienna, 1975), Vol. 2, p. 419.
J. F. Counsell, J. F. Martin, R. M. Dell, and A. R. Junkison, in Thermodynamics of Nuclear Materials (IAEA, Vienna, 1967), p. 385.
A. Blaise, J. Phys. (Paris) 40 (Coll. 4), C4–49 (1979).
R. Troć and Z. Kletowski, Bull. Acad. Polon. Sci. Ser. Sci. Chim. 22, 621 (1974).
P. J. Markowski, A. Blaise, and Z. Henkie, Rocz. Chem. 51, 1027 (1977).
O. L. Kruger and J. B. Moser, J. Phys. Chem. Solids 28, 2321 (1967).
R. Benz, J. Nucl. Mat. 25, 233 (1968).
J. Leciejewicz, A. Murasik, and R. Troć, Phys. Stat. Sol. 30, 157 (1968).
G. H. Lander, M. H. Mueller, and J. F. Reddy, Phys. Rev. B 6, 1880 (1972).
A. Murasik, J. Leciejewicz, H. Ptasiewicz-Bak, R. Troć, A. Zygmunt, and Z. Zoŀnierek, in Proc. 2nd Intern. Conf. on the Electronic Structure of the Actinides, J. Mulak, W. Suski, and R. Troć, eds. (Ossolineum, Wrocŀaw, 1977), p. 405.
H. Bartholin and O. Vogt, Private communication.
J. A. C. Marples, C. F. Sampson, S. A. Wedgwood, and M. Kuznietz, J. Phys. C: SolidState Phys. 8, 708 (1975).
M. Obolenski and R. Troć, in Proc. 2nd Int. Conf. on the Electronic Structure of the Actinides, J. Mulak, W. Suski, and R. Troć, eds. (Ossolineum, Wrocław, 1977), p. 397.
A. Furrer, A. Murasik, and O. Vogt, Helv. Phys. Acta 5, 447 (1977).
W. Trzebiatowski, A. Sepichowska, and A. Zygmunt, Bull. Acad. Polon. Sci. Ser. Sci. Chim. 14, 495 (1966).
R. Lagnier, J. Pierre, and M. J. Mortimer, Cryogenics 17, 349 (1967).
V. Maurice, J. L. Boutard, and D. Abbe, J. Phys. (Paris) 40 (Coll. 4), C4–140 (1979).
R. Troć and D. J. Lam, Phys. Stat. Sol. (b) 65, 317 (1974).
R. Ramji Rao and J. V. S. S. Narayana Murty, J. Low. Temp. Phys. 33, 413 (1978).
E. F. Westrum, Jr., R. R. Walters, H. E. Flotow, and D. W. Osborne, J. Chem. Phys. 48, 155 (1968).
H. F. Flotow, D. W. Osborne, and R. R. Walters, J. Chem. Phys. 55, 880 (1971).
R. J. Trainor, M. B. Brodsky, and G. S. Knapp, in Plutonium and Other Actinides, H. Blank and R. Lindner, eds. (North-Holland, Amsterdam, 1976), p. 475.
M. Steinitz and J. Grunzweig-Genossar, J. Phys. (Paris) 40 (Coll. 4), C4–34 (1979).
J. Danan, C. H. de Novion, Y. Guerin, and F. A. Wedgwood, J. Phys. (Paris) 37, 1169 (1976).
G. H. Lander, Private communication.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Blaise, A., Troć, R., Lagnier, R. et al. The heat capacity of uranium monoarsenide. J Low Temp Phys 38, 79–92 (1980). https://doi.org/10.1007/BF00115269
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00115269