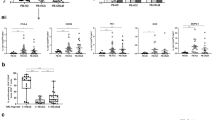
The liver is the most common site of hematogenous metastases from colorectal carcinoma. Kupffer cells (KC), which line the hepatic sinusoids, may form the first line of defense against circulating tumor cells. The purpose of this study was to determine the effect of hepatic metastases and intro-abdominal tumor growth on KC binding of human colorectal carcinoma (HCRC) cells. MIP-101, a poorly metastatic cell line, and CX-1, a highly metastatic cell line, were injected intrasplenically into nude mice and KC were isolated by collagenase perfusion at varying intervals after injection. Conditioned media were collected from MIP-101, CCL 188 and CX-1 to determine their in vitro effect on KC function. KC from MIP-101 injected mice (14% liver metastases, 100% splenic tumors) bound a significantly greater number of MIP-101 and clone A cells than CX-1 cells in vitro. KC isolated from mice 5 weeks after CX-1 injection (100% liver metastases) also showed increased binding of MIP-101 and clone A cells compared to CX-1 cells. Similar results were obtained when tumor cell binding to normal human liver KC was compared to binding to KC from human livers from patients with hepatic metastasis from colorectal cancer. In contrast KC obtained from mice 3 weeks after CX-1 injection (44% liver metastases) showed significantly decreased binding of MIP-101 and clone A cells. The conditioned medium from CX-1 cells significantly decreased the in vitro binding of both MIP-101 and CX-1 by KC. These results indicate that the ability of KC to bind HCRC cells (which precedes phagocytosis and tumor cell killing) is a dynamic function and affected by concomitant tumor growth. HCRC cells may alter KC function via the production of specific tumor-derived soluble factors. In order to devise new and more effective therapeutic options in the treatment of liver metastases the nature of this tumor cell-KC interaction must be better understood.
Similar content being viewed by others
References
Cohen AM, Shank B and Friedman MA. Colorectal cancer. In: DeVita VT, Hellman S and Rosenberg S, eds. Cancer: principles and practice of oncology, p. 907. Pennsylvania: J.B. Lippincott Company, 1989.
Silverberg E and Lubera J, 1989, Cancer statistics. Cancer, 37, 2–19.
Liotta L and Stetler-Stevenson WG, 1991, Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Research, (Suppl) 51, 50545s–505549s.
Phillips NC, 1989, Kupffer cells and liver metastases. Cancer Metastases Reviews, 8, 231–252.
Pearson HJ, Anderson J, Chamberlain J and Bell PRF, 1986, The effect of Kupffer cell stimulation or depression on the development of liver metastases in the rat. Cancer Immunology and Immunotherapy, 23, 214–216.
Thombre PS and Deodhar S, 1984, Inhibition of liver metastases in murine colon adenocarcinoma by liposomes containing human c-reactive protein or crude lymphokine. Cancer Immunology and Immunotherapy, 16, 145–150.
Nelson DS and Nelson M, 1987, Evasion of host defenses by tumor. Immunology and Cell Biology, 65, 287–304.
Tsunavaki SM, Sporn M, Ding A and Nathan C, 1988, Deactivation of macrophages by transforming growth factor β. Nature, 334, 260–262.
Parhar RS and Lala PK, 1988, Prostaglandin E2 mediated inactivation of various killer lineage cells by tumor bearing host macrophages. Journal of Leucocyte Biology, 44, 474.
Lopez DM, Lopez-Ceparo M, Watson GA, Ganju A, Sotomayor E and Fu Y-X, 1991, Modulation of the immune system by mammary tumor-derived factors. Cancer Investigations, 9, 641–653.
Niles R, Wilhelm S, Steele G, Burke B, Christensen T, Dexter D, O'Brien MS, Thomas P and Zamcheck N, 1987, Isolation and characterization of an undifferentiated human colon carcinoma cell line (MIP101). Cancer Investigation, 5, 545–552.
12. Wagner HE, Toth CA, Steele GD and Thomas P, 1992, Metastatic potential of human colon cancer cell lines: relationship to cellular differentiation and carcinoembryonic antigen production. Clinical and Experimental Metastasis, 10, 25–31.
Giavazzi R, Jessup J, Campbell D, Walker S and Fidler I, 1986, Experimental nude mouse model of human colorectal cancer liver metastases. Journal of the National Cancer Institute, 77, 1303–1308.
Toth CA, Thomas P, Broitman SA and Zamcheck N, 1985, Receptor mediated endocytosis of carcinoembryonic antigen by rat liver Kupffer cells. Cancer Research, 45, 392–397.
Reske SN, Nyska K and Feinendegen LE, 1981, In-vivo assessment of phagocytic properties of Kupffer cells. Journal of Nuclear Medicine, 22, 405–410.
Frenzel H, Kremer B and Hacher H. The liver sinusoids under various pathological conditions: a TEM and SEM study of rat liver after respiratory hypoxia, telecobalt irradiation and endotoxin application. In: Wisse E and Knook DL, eds. Kupffer Cells and Other Liver Sinusoidal Cells, pp. 213–222. Amsterdam: Elsevier, North Holland Biomedical Press, 1987.
Roerdink FH, Wisse E, Movelt H, Van der Meulen J and Scherphof GL. Cellular distribution of intravenously injected protein containing liposomes in the rat liver. In: Wisse E and Knook DL, eds. Kupffer Cells and Other Liver Sinusoidal Cells, pp. 262–272. Amsterdam: Elsevier, North Holland Biomedical Press, 1977.
Gardner CR, Wasserman AJ and Laskin DL, 1987, Differential sensitivity of tumor targets to liver macrophage-mediated cytotoxicity. Cancer Research, 47, 6686–6691.
Gjoen T, Seljelid R and Kolset SO, 1989, Binding of metastatic colon carcinoma cells to liver macrophages. Journal of Leucocyte Biology, 45, 362–369.
Williams DL, Sherwood ER, McNamee RB, Jones EL and Diluzio NR, 1985, Therapeutic efficacy of glucan in a murine model of hepatic metastatic disease. Hepatology, 5, 198–206.
Thombre PS and Deodhar SD, 1984, Inhibition of liver metastases in murine colon adenocarcinoma by liposomes containing c-reactive protein or crude lymphokine. Cancer Immunology and Immunotherapy, 16, 145–150.
Brodt P, Blore J, Phillips NC, Meinger JS and Rioux JD, 1981, Inhibition of murine hepatic tumor growth by liposomes containing a lipophilic muramyl dipeptide. Cancer Immunology and Immunotherapy, 28, 54–58.
Kopper L, Van Hank T and Lapes K, 1982, Experimental model for liver metastasis using Lewis lung tumor. Journal of Cancer Research and Clinical Oncology, 103, 31–38.
Chattopadhyay U, Bhattacharrya S and Chaktabarty NG, 1986, Tumor-associated macrophage mediated lysis of autologous tumor cells. Neoplasma, 33, 157–165.
Duffie GP and Young RI, 1991, Tumoricidal activity of alveolar and peritoneal macrophages of C57BL/6 mice bearing metastatic or nonmetastatic variants of Lewis lung carcinoma. Journal of Leucocyte Biology, 49, 8–14.
Taffet SM and Russell SW, 1981, Macrophage-mediated tumor cell killing: regulation of expression of cytolytic activity by prostaglandin E. Journal of Immunology, 126, 424–427.
Pelus LM and Bockman RS, 1979, Increased prostaglandin synthesis by macrophages from tumor-bearing mice. Journal of Immunology, 123, 2118.
Utsugi T and Fidler IJ, 1991, Prostaglandin E2 does not inhibit tumoricidal activity of mouse macrophages against adherent tumor cells. Journal of Immunology, 146, 2066–2071.
Sotomayor E, Fu X-Y, Lopez-Ceparo M, Herbert L, Jimenez JJ, Albaracin C and Lopez DM, 1991, Role of tumor-derived cytokines on the immune system of mice bearing a mammary adenocarcinoma: 11 Down-regulation of macrophage-mediated cytotoxicity by tumor-derived granulocyte macrophage colony stimulating factor. Journal of Immunology, 8, 2816–2823.
Evans R, Kamdar SJ and Duffy TM, 1991, Tumor-derived products induce Il-1a, Il-1b, TNFa and IL 6 gene expression in murine macrophages: distinctions between tumor and bacterial endotoxin induced gene expression. Journal of Leucocyte Biology, 49, 474–482.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meterissian, S., Steele, G.D. & Thomas, P. Human and murine Kupffer cell function may be altered by both intrahepatic and intrasplenic tumor deposits. Clin Exp Metast 11, 175–182 (1993). https://doi.org/10.1007/BF00114975
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00114975