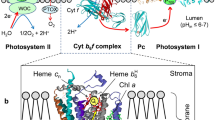
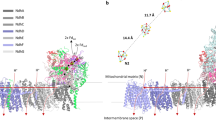
Abstract
It has been known for some time that bicarbonate reverses the inhibition, by formate under HCO3 --depletion conditions, of electron transport in thylakoid membranes. It has been shown that the major effect is on the electron acceptor side of photosystem II, at the site of plastoquinone reduction. After presenting a historical introduction, and a minireview of the bicarbonate effect, we present a hypothesis on how HCO3 - functions in vivo as (a) a proton donor to the plastoquinone reductase site in the D1-D2 protein; and (b) a ligand to Fe2+ in the QA-Fe-QB complex that keeps the D1-D2 proteins in their proper functional conformation. They key points of the hypothesis are: (1) HCO3 - forms a salt bridge between Fe2+ and the D2 protein. The carboxyl group of HCO3 - is a bidentate ligand to Fe2+, while the hydroxyl group H-bonds to a protein residue. (2) A second HCO3 - is involved in protonating a histidine near the QB site to stabilize the negative charge on QB. HCO3 - provides a rapidly available source of H+ for this purpose. (3) After donation of a H+, CO3 2- is replaced by another HCO3 -. The high pKa of CO3 2- ensures rapid reprotonation from the bulk phase. (4) An intramembrane pool of HCO3 - is in equilibrium with a large number of low affinity sites. This pool is a H+ buffering domain functionally connecting the external bulk phase with the quinones. The low affinity sites buffer the intrathylakoid [HCO3 -] against fluctuations in the intracellular CO2. (5) Low pH and high ionic strength are suggested to disrupt the HCO3 - salt bridge between Fe2+ and D2. The resulting conformational change exposes the intramembrane HCO3 - pool and low affinity sites to the bulk phase.
Two contrasting hypotheses for the action of formate are: (a) it functions to remove bicarbonate, and the low electron transport left in such samples is due to the left-over (or endogenous) bicarbonate in the system; or (b) bicarbonate is less of an inhibitor and so appears to relieve the inhibition by formate. Hypothesis (a) implies that HCO3 - is an essential requirement for electron transport through the plastoquinones (bound plastoquinones QA and QB and the plastoquinone pool) of photosystem II. Hypothesis (b) implies that HCO3 - does not play any significant role in vivo. Our conclusion is that hypothesis (a) is correct and HCO3 - is an essential requirement for electron transport on the electron acceptor side of PS II. This is based on several observations: (i) since HCO3 -, not CO2, is the active species involved (Blubaugh and Govindjee 1986), the calculated concentration of this species (220 μM at pH 8, pH of the stroma) is much higher than the calculated dissociation constant (Kd) of 35–60 μM; thus, the likelihood of bound HCO3 - in ambient air is high; (ii) studies on HCO3 - effect in thylakoid samples with different chlorophyll concentrations suggest that the “left-over” (or “endogenous”) electron flow in bicarbonate-depleted chloroplasts is due to “left-over” (or endogenous) HCO3 - remaining bound to the system (Blubaugh 1987).
Similar content being viewed by others
Abbreviations
- DCMU:
-
3-(3,4-dichlorophenyl)-1, 1-dimethylurea (common name: diuron)
- PSII:
-
photosystem II
- QA :
-
first plastoquinone electron acceptor of PSII
- QB :
-
second plastoquinone acceptor of PS II
References
Abeles, FB, Brown, AH, Mayne, BC (1961) Stimulation of the Hill reaction by carbon dioxide. Plant Physiol 36: 202–207.
Babcock, GT (1987) The photosynthetic oxygen evolving process. In: Amesz J (ed.) Photosynthesis, Chapte 6, pp 125–158. Elsevier Science Publishers B.V. (Biomed Div).
Barnard, EA and Stein, WD (1959) The histidine residue in the active centre of ribonuclease: I. A specific reaction with bromoacetic acid. J Mol Biol 1: 339–349.
Barr, R, and Crane, FL (1976) Control of photosynthesis by CO2: evidence for a bicarbonate-inhibited redox feedback in photosystem II. Proc Indiana Acad Sci 85: 120–128.
Batra, PP and Jagendorf, AT (1965) Bicarbonate effects on the Hill reaction and photophosphorylation. Plant Physiol 40: 1074–1079.
Blubaugh DJ (1987) The mechanism of bicarbonate activation of plastoquinone reduction in photosystem II of photosynthesis. Ph. D. Thesis, University of Illinois at Urbana-champaign.
Blubaugh, DJ and Govindjee (1984) Comparison of bicarbonate effects on the variable chlorophyll a fluorescence of CO2-depleted and non-CO2-deleted thylakoids in the presence of Diuron. Z Naturforsch 39c: 378–381.
Blubaugh, DJ and Govindjee (1986) Bicarbonate, not CO2, is the species required for the stimulation of photosystem II electron transport. Biochim Biophys Acta 848 147–152.
Blubaugh DJ and Govindjee (1988b) Sites of inhibition by disulfiram in thylakoid membranes. Plant Physiol, in press.
Böger, P (1982) Replacement of photosynthetic electron transport inhibitors by silicomolybdate. Physiol plant 54: 221–224.
Bowes, JM, Crofts, AR and Itoh, S (1979) A high potential acceptor for photosystem II. Biochim Biophys Acta 547: 320–335.
Boyle, FP (1948) Some factors involved in oxygen evolution from triturated spinach leaves. Science 108: 359–360.
Brown, AH and Franck, J (1948) On the participation of carbon dioxide in the photosynthetic activity of illuminated chloroplast suspensions. Arch Biochem 16: 55–60.
Burk, D and Warburg, O (1950) l-Quanten-Mechanismus und Energie-Kreisprozess bei der Photosynthese. Die Naturwissenschaften 37: 560–569.
Cohen, WS and MacPeek, WA (1980) A proposed mechanism for the stimulatory effect of bicarbonate ions on ATP synthesis in isolated chloroplasts. Plant Physiol 66: 242–245.
Critchley, C, Baianu, IC, Govindjee and Gutowsky, HS (1982) The role of chloride in O2 evolution by thylakoids from salt-tolerant higher plants. Biochim Biophys Acta 682: 436–445.
Crofts, AR, Robinson, HH and Snozzi, M (1984) Reactions of quinones at catalytic sites; a diffusional role in H-transfer. In: C, Sybesma (ed.) Advances in Photosynthesis Research, pp461–468 The Hague: Martinus Nijhoff.
Diner, BA (1977) Dependence of the deactivation reaction of photosystem II on the redox state of plastoquinone pool A, varied under anaerobic conditions: equilibria on the acceptor side of photosystem II. Biochim Biophys Acta 460: 247–258.
Dole, M (1935) The relative atomic weight of oxygen in water and in air. J Am Chem Soc 57:2731–2731.
Dole, M and Jenks, G (1944) Isotopic composition of photosynthetic oxygen. Science 100: 409–409.
Duysens, LNM and Sweers, HE (1963) Mechanism of two photochemical reactions in algae as studied by means of fluorescence. In: Jap Soc of Plant Physiol (eds.) Studies on Microalgae and Photosynthetic Bacteria, pp 353–372. Tokyo: The University of Tokyo Press.
Eaton-Rye JJ (1987) Bicarbonate reversible anionic inhibition of the quinone reduetase in photosystem II. Ph.D. Thesis. University of Illinois at Urbana.
Eaton-Rye, JJ and Govindjee (1984) A study of the specific effect of bicarbonate on photosynthetic electron transport in the presence of methyl viologen. Photobiochem Photobiophys 8: 279–288.
Eaton-Rye, JJ, Blubaugh, DJ and Govindjee (1986) Action of bioarbonate on photosynthetic electron transport in the presence or absence of inhibitory anions. In: GC, Papageorgiou, J, Barber and S, Sapa (eds) Ion Interactions in Energy Transfer Biomembranes pp 263–278. New York: Plenum Publishing Corporation.
Farineau, J and Mathis, P (1983) Effect of bicarbonate on electron transfer between plastoquinones in photosystem II. In: Y, Inoue, AR, Crofts, Govindjee, N, Murata, G, Renger and K, Satoh (eds) The Oxygen-Evolving System of Plant Photosynthesis, pp 317–325. New York: Academic Press.
Fischer, K and Metzner, H (1981) Bicarbonate effects on photosynthetic electron transport: I Concentration dependence and influence on manganese reincorporation. Photobiochem Photobiophys 2: 133–140.
Förster, V, Hong, Y-Q and Junge, W (1981) Electron transfer and proton pumping under excitation of dark-adapted chloroplasts with flashes of light. Biochim Biophys Acta 638: 141–152.
Foster, JW (1940) The role of organic substrates in photosynthesis of purple bacteria. J Gen Physiol 24: 123–134.
Fowler, CF (1977) Proton translocation in chloroplasts and its relationship to electron transport between the photosystems. Biochim Biophys Acta 459: 351–363.
Franck, J (1945) Photosynthetic activity of isolated chloroplasts. Rev Mod Phys 17: 112–119.
French, CS (1937) The quantum yield of hydrogen and carbon dioxide assimilation in purple bacteria. J Gen Physiol 20: 711–735.
French, CS, Holt, AS, Powell and Anson, HL (1946) The evolution of oxygen from illuminated suspensions of frozen, dried and homogenized chloroplasts. Science 103: 505–506.
Gaffron, H (1940) Carbon dioxide reduction with molecular hydrogen in green algae. Am J Bot 27: 273–283.
Garab, G, Sanchez Burgos, AA, Zimányi, L and Faludi-Dánlel, A (1983) Effect of CO2 on the energization of thylakoids in leaves of higher plants. FEBS Lett 154: 323–327.
Garab G, Chylla RG and Whitmarsh J (1987) Photosystem II: evidence for active and inactive complexes. In: M Gibbs (ed) Hungarian-USA Binational Symposium on Photosynthesis, pp 37–47.
Giaquinta, RT and Dilley, RA (1975) A partial reaction in photosystem II: reduction of silicomolybdate prior to the site of dichlorophenyl-dimethylurea inhibition. Biochim Biophys Acta 387: 288–305.
Gibbons, BH and Edsall, JT (1963) Rate of hydration of carbon dioxide and dehydration of carbonic acid at 25°C. J Biol Chem 238: 3502–3507.
Good, NE (1963) Carbon dioxide and the Hill reaction. Plant Physiol 38: 298–304.
Govindjee (ed.) Bioenergetics of Photosynthesis. New York: Academic Press.
Govindjee (ed.) (1982) Photosynthesis, Vol 1. Energy Conversion by Plants and Bacteria, New York: Academic Press.
Govindjee and Eaton-Rye, JJ (1986) Electron transfer through photosystem II acceptors: interaction with anions. Photosynth Res 10: 365–379.
Govindjee and van, Rensen, JJS (1978) Bicarbonate effects on the electron flow in isolated broken chloroplasts. Biochim Biophys Acta 505: 183–213.
Govindjee, Pulles, R, Govindjee, R, van, Gorkom and Duysens, LNM (1976) Inhibition of the reoxidation of the secondary electron acceptor of photosystem II by bicarbonate depletion. Biochim Biophys Acta 449: 602–605.
Govindjee, Nakatani, HY, Rutherford, AW and Inoue (1984) Evidence from thermoluminesence for bicarbonate action on the recombination reactions involving the secondary quinone electron acceptor of photosystem II. Biochim Biophys Acta 766: 416–423.
Govindjee, Kambara, T and Coleman, W (1985) The electron donor side of photosystem II: the oxygen evolving complex. Photochem Photobiol 42: 187–210.
Govindjee, Amesz, J and Fork, DC (ed) (1986) Light Emission by Plants and Bacteria, Orlando FL: Academic Press.
Graan, T (1986) The interaction of silicomolybdate with the photosystem II herbicide-binding site. FEBS Lett 206: 9–14.
Graan, T and Ort, DR (1986) Detection of oxygen evolving photosystem II centers inactive in plastoquinone reduction. Biochim Biophys Acta 852: 320–330.
Greene, CH and Voskuyl, RJ (1936) An explanation of the relatively large concentration of 18O in the atmosphere. J Am Chem Soc 58: 693–694.
Harnischfeger, G (1974) Studies on the effect of diphenylcarbazide in isolated chloroplasts from spinach. Z. Naturforsch 29c: 705–709.
Hesketh, JD, Woolley, JT and Peters, DB (1982) Predicting Photosynthesis. In: Govindjee (ed) Photosynthesis, Vol. II, Development, Carbon Metabolism, and Plant Productivity, pp 387–418. New York: Academic Press.
Hill, R (1937) Oxygen evolved by isolated chloroplasts. Nature (London) 139: 881–882.
Hill, R (1939) Oxygen produced by isolated chloroplasts. Proc Roy Soc London B 127: 192–210.
Hill, R and Bendell, F (1960) Function of the two cytochrome components in chloroplasts: a working hypothesis. Nature (London) 186: 136–137.
Hill, R and Scarisbrick, R (1940a) Production of oxygen by illuminated chloroplasts. Nature (London) 146: 61–62.
Hill, R and Scarisbrick, R (1940b) The reduction of ferric oxalate by isolated chloroplasts. Proc Roy Soc London B 129: 238–255.
Hope, AB and Moreland, A (1979) Proton translocation in isolated spinach chloroplasts after single-turnover actinic flashes. Aust J Plant Physiol 6: 289–304.
Ikegami I and Katoh S (1973) Studies on chlorophyll fluorescence in chloroplasts: II. Effect of ferricyanide on the induction of fluorescence in the presence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea Plant Cell Physiol 829–836.
Ireland, CR, Baker, NR and Long, SP (1987) Evidence for a physiological role of CO2 in the regulation of photosynthetic electron transport in intact leaves. Biochim Biophys Acta 893: 434–443.
Itoh, S (1978) Membrane surface potential and the reactivity of the system II primary electron acceptor to charged electron carriers in the medium. Biochim Biophys Acta 504: 324–340.
Itoh, S and Nishimura, M (1977) pH dependent changes in the reactivity of the primary electron acceptor of system II in spinach chloroplasts to external oxidant and reductant. Biochim Biophys Acta 460: 381–392.
Izawa, S (1962) Stimulatory effect of carbon dioxide upon the Hill reaction as observed with the addition of carbonic anhydrase to reaction mixture. Plant Cell Physiol 3: 221–227.
Jursinic, P and Stemler, A (1982) A seconds range component of the reoxidation of the primary photosytem II acceptor, Q: effects of bicarbonate depletion in chloroplasts. Biochim Biophys Acta 681: 419–428.
Jursinic, P and Stemler, A (1986) Correlation between the binding of formate and decreased rates of charge transfer through the photosystem II quinones. Photochem Photobiol 43: 205–212.
Jursinic, P and Stemler, A (1988) Multiple anion effects on photosystem II in chloroplast membranes. Photosynth Res 15: 41–56.
Jursinic, P, Warden, J and Govindjee (1976) A major site of bicarbonate effect in system II reaction: evidence from ESR signal IIvf, fast fluorescence yield changes and delayed light emission. Biochim Biophys Acta 440: 322–330.
Kamen, MD and Barker, HA (1945) Inadequacies in present knowledge of the relation between photosynthesis and 18O content of atmoshperic oxygen. Proc Natl Acad Sci USA 31: 8–15.
Khanna, R, Govindjee and Wydrzynski (1977) Site of bicarbonate effect on Hill reaction: evidence from the use of artificial electron acceptors and donors. Biochim Biophys Acta 462: 208–214.
Khanna, R, Pfister, K, Keresztes, A, van, Rensen, JJS and Govindjee (1981) Evidence for a close spatial location of the binding sites for CO2 and for photosystem II inhibitors. Biochim Biophys Acta 634: 105–116.
Korman, S and Clarke, HT (1955) Carboxymethylamino acids and peptides. J Biol Chem 221: 113–131.
Lorimer, GH, Badger, MR and Andrews, TJ (1976) The activation of ribulose-1,5,-bisphos-phate carboxylase by carbon dioxide and magnesium irons: equilibria, kinetics, a suggested mechanism, and physiological implications. Biochem 15: 529–536.
Metzner, H (1975) Water decomposition in photosynthesis? A critical reconsideration. J Theor Biol 51: 201–231.
Michel, H and Deisenhofer, J (1988) Relevance of the photosynthetic reaction center from purple bacteria to the structure of photosystem II. Biochemistry 27: 1–7.
Michel, H, Epp, O and Deisenhofer, J (1986) Pigment-protein interactions in the photosynthetic reaction centre from Rhodopseudomonas viridis. EMBO J 5: 2445–2451.
Mills, A and Urey, HC (1940) The kinetics of isotopic exchange between carbon dioxide, bicarbonate ion, carbonate ion and water. J Am Chem Soc 62: 1019–1026.
Murata, N, Nishimura, M and Takamiya, A (1966) Fluorescence of chlorophyll in photosynthetic systems: II. Induction of fluorescence in isolated spinach chloroplasts. Biochim Biophys Acta 120: 23–33.
Nelson, N, Nelson, H and Racker, E (1972) Partial resolution of the enzymes catalyzing photophosphorylation: XI. Magnesium-adenosine triphosphatase properties of heat-activated coupling Factor 1 from chloroplasts. J. Biol Chem 247: 6505–6510.
Oettmeier, W and Soll, HJ (1983) Competition between plastoquinone and 3-(3,4-dichlorophenyl)-1,1-dimethylurea at the acceptor side of photosystem II. Biochim Biophys Acta 724: 287–290.
Pearlstein, RM (1982) Chlorophyll singlet excitons. In: Govindjee (ed.) Photosynthesis, Vol. 1. Energy Conversion by Plants and Bacteria, pp 293–330. New York: Academic Press.
Petroules, V and Diner, B (1986) Identification of Q400, a high potential electron acceptor of photosystem II, the iron of the quinone-iron acceptor complex. Biochim Biophys Acta 849: 264–275.
Pulles, MPJ, van, Gorkom, HJ and Willemsen, JG (1976) Absorbance changes due to the charge-accumulating species in system 2 of photosynthesis. Biochim Biophys Acta 449: 536–540.
Punnett, T and Iyer, RV (1964) The enhancement of photophosphorylation and the Hill reaction by carbon dioxide. J Biol Chem 239: 2335–2339.
Rabinowitch, E (1945, 1951, 1956) Photosynthesis and Related Processes, Vol. I, pp 54–56, 61–67; Vol II (parts 1 and 2), pp 1529–1530, 1915–1918 (part 2) New York: Interscience.
Radmer, R and Ollinger, O (1980) Isotopic composition of photosynthetic O2 flash yields in the presence of H2 18O and HC18O3 -. FEBS Lett 110: 57–61.
Renger, G (1976) Studies on the structural and functional organization of system II of photosynthesis: the use of trypsin as a structurally selective inhibitor at the outer surface of the thylakoid membrane. Biochim Biophys Acta 440: 203–224.
Renger, G (1987) Mechanistic aspects of photosynthetic water cleavage. Photosynthetica 21: 203–224.
Renger, G and Govindjee (1985) The mechanism of photosynthetic water oxidation. Photosynth Res 6: 33–55.
Robinson, HH, Eaton-Rye, JJ, van, Rensen, JJS and Govindjee (1984) The effects of bicarbonate depletion and formate incubation on the kinetics of oxidation-reduction reactions of the photosystem II quinone acceptor complex. Z Naturforsch 39c: 382–385.
Ruben, S, Randall, M, Kamen, MD and Hyde, JL (1941) Heavy oxygen (18O) as a tracer in the study of photosynthesis. J Am Chem Soc 63: 877–878.
Rutherford, AW and Zimmermann, JL (1984) A new EPR signal attributed to the primary plastoquinone acceptor in photosystem II. Biochim Biophys Acta 767: 168–175.
Sarojini, G and Govindjee (1981a) On the active speices in bicarbonate stimulation of Hill reaction in thylakoid membranes. Biochim Biophys Acta 634: 340–313.
Sarojini, G and Govindjee (1981b) Is CO2 an active species in stimulating the Hill reaction in thylakoid membranes? In: G., Akoyunoglou (ed.) Photosynthesis, Vol. 2, Electron Transport and Photophosphorylation, pp. 143–149. Philadelphia: Balaban International Science Services.
Shipman, LL (1981) Theoretical study of the binding site and mode of action for photosystem II herbicides. J. Theor Biol 90: 123–148.
Siggel, U, Khanna, R, Renger, G and Govindjee (1977) Investigation of the absorption changes of the plastoquinone system in broken chloroplasts: the effect of bicarbonate-depletion. Biochim Biophys Acta 462: 196–207.
Snel, JFH and van, Rensen, JJS (1983) Kinetics of the reactivation of the Hill reaction in CO2-depleted chloroplasts by addition of bicarbonate in the absence and in the presence of herbicides. Physiol Plant 57: 422–427.
Snel, JFH and van, Rensen, JJS (1984) Reevaluation of the role of bicarbonate and formate in the regulation of photosynthetic electron flow in broken chloroplasts. Plant Physiol 75: 146–150.
Stemler, A (1977) The binding of bicarbonate ions to washed chloroplast grana. Biochim Biophys Acta 460: 511–522.
Stemler, A (1979) A dynamic interaction between the bicarbonate ligand and photosystem II reaction center complexes in chloroplasts. Biochim Biophys Acta 545: 36–45.
Stemler, A (1980) Forms of dissolved carbon dioxide required for photosystem II activity in chloroplast membranes. Plant Physiol 65: 1160–1165.
Stemler, A (1982) The functional role of bicarbonate in photosynthetic light reaction II. In Govindjee (ed.) Photosynthesis, Vol. II. Development, Carbon Metabolism, and Plant Productivity, pp 513–558. New York: Academic Press.
Stemler A (1985) Carbonic anhydrase: Molecular insights applied to photosystem II research in thylakoid membranes. In: WJ Lucas and JA Berry (eds.) Inorganic Carbon Uptake by Aquatic Photosynthetic Organisms. American Society of Plant Physiologists, pp 377–387.
Stemler, A and Govindjee (1973) Bicarbonate ion as a critical factor in photosynthetic oxygen evolution. Plant Physiol 52: 119–123.
Stemler, A and Govindjee (1974) Effects of bicarbonate ion on chlorophyll a fluorescence transients and delayed light emission from maize chloroplasts. Photochem Photobiol 19: 227–232.
Stemler, A and Murphy, J (1983) Determination of the binding constant of H14CO3 - to the photosystem II complex in maize chloroplasts: effects of inhibitors and light. Photochem Photobiol 38: 701–707.
Stemler, A and Murphy, J (1984) Inhibition of HCO3 - binding to photosystem II by atrazine at a low-affinity herbicide binding site. Plant Physiol 76: 179–182.
Stemler, A and Radmer, R (1975) Source of photosynthetic oxygen in bicarbonate-stimulated Hill reaction. Science 190: 457–458.
Stemler, A, Babeock, GT and Govindjee (1974) The effect of bicarbonate on photosynthetic oxygen evolution in flashing light in chloroplast fragments. Proc Nat Acad Sci USA 71: 4679–4683.
Stern, BK and Vennesland, B (1962) The effect of carbon dioxide on the Hill reaction. J Biol Chem 237: 596–602.
Stiehl, HH and Witt, HT (1969) Quantitative treatment of the function of plastoquinone in photosynthesis. Z Naturforsch 24b: 1588–1598.
Trebst, A (1987) The three-dimensional structure of the herbicide binding niche on the reaction center polypeptides of photosystem II. Z Naturforsch 42c: 742–750.
Trebst, A and Draber, W (1986) Inhibitors of photosystem II and the topology of the herbicide and QB binding polypeptide in the thylakoid membrane. Photosynth Res 10: 381–392.
van, Grondelle, R and Amesz, J (1986) Excitation energy transfer in photosynthetic systems. In: Govindjee, J, Amesz and DC, Fork (eds.) Light Emission by Plants and bacteria, pp 191–224. Orlando: Academic Press.
van, Niel, CB (1931) On the morphology and physiology of the purple and green sulphur bacteria. Arch Microbiol 3: 1–102.
van, Niel, CB (1941) The bacterial photosynthesis and their importance for the general problem of photosynthesis. Adv Enzymol 1: 263–328.
van, Niel, CB (1949) The comparative biochemistry of photosynthesis. In J, Franck, WE, Loomis (eds.) Photosynthesis in Plants pp 437–495. Ames, Iowa: Iowa State College Press.
van, Rensen, JJS (1988) Involvement of bicarbonate in the protonation of the secondary quinone electron acceptor of photosystem II via the non-haem iron of the quinone-iron acceptor complex. FEBS Lett 226: 347–351.
van, Rensen, JJS and Snel, JFH (1985) Regulation of photosynthetic electron transport by bicarbonate, formate, and herbicides in isolated broken and intact chloroplasts. Photosynth Res 6: 231–246.
van, Rensen, JJS and Vermaas, WEJ (1981) Action of bicarbonate and photosystem 2 inhibiting herbicides on electron transport in pea grana and in thylakoids of a blue-green alga. Physiol Plant 51: 106–110.
Velthuys, BR (1981) Electron-dependent competition between plastoquinone and inhibitors for binding to photosystem II. FEBS Lett 126: 277–281.
Vermaas WFJ (1984) The interaction of quinones, herbicides and bicarbonate with their binding environment at the acceptor side of photosystem II in photosynthesis. Ph.D. Thesis, Agricultural University Wageningen, The Netherlands.
Vermaas, WFJ and Govindjee (1981a) Unique role(s) of carbon dioxide and bicarbonate in the photosynthetic electron transport system. Proc Indian Nat Sci Acad B 47: 581–605.
Vermaas, WFJ and Govindjee (1981b) The acceptor side of photosystem II in photosynthesis. Photochem Photobiol 34: 775–793.
Vermaas, WFJ and Govindjee (1982a) Bicarbonate or CO2 as a requirement for efficient electron transport on the acceptor side of photosystem II. In: Govindjee (ed.) Photosynthesis, Vol. II. Development, Carbon Metabolism, and Plant Productivity, pp 541–558. New York: Academic Press.
Vermaas, WFJ and Govindjee (1982b) Bicarbonate effects on chlorophyll a fluorescence transients in the presence and absence of diuron. Biochim Biophys Acta 680: 202–209.
Vermaas, WFJ and Rutherford, AW (1984) EPR measurements on the effects of bicarbonate and triazine resistance on the acceptor side of photosystem II. FEBS Lett 175: 243–248.
Vermaas, WFJ and van, Rensen, JJS (1981) Mechanism of bicarbonate action on photosynthetic electron transport in broken chloroplasts. Biochim Biophys Acta 636: 168–174.
Vermaas, WFJ, van, Rensen, JJS and Govindjee (1982) The interaction between bicarbonate and the herbicide ioxynil in the thylakoid membrane and the effects of amino acid modification on bicarbonate action. Biochim Biophys Acta 681: 242–247.
Vermaas, WFJ, Arntzen, CJ, Gu, L-Q and Yu, C-A (1983) Interactions of herbicides and azidoquinones at a photosystem II binding site in the thylakoid membrane. Biochim Biophys Acta 723: 266–275.
Warburg, O (1964) Prefatory chapter. Ann Rev Biochem 33: 1–18.
Warburg, O and Krippahl, G (1958) Hill-Reaktionen. Z Naturforsch 13b: 509–514.
Warburg, O and Krippahl, G (1960) Notwendigkeit der Kohlensaure für die chinon und ferricyanid-Reactionen in grünen Grana. Z Naturforsch 15b: 367–369.
Warburg, O, Krippahl, G, Gewitz, HS and Volker, W (1959) Uber den chemischen Mechanismus der Photosynthese. Z Naturforsch 14b: 712–724.
Webster, LA, Wahl, MH and Urey, HC (1935) The fractionation of the oxygen isotopes in an exchange reaction. J. Chem Phys 3: 129–129.
Wraight, CA (1979) Electron acceptors of bacterial photosynthetic reaction centers: II. H+ binding coupled to secondary electron transfer in the quinone acceptor complex. Biochim Biophys Acta 548: 309–327.
Wraight, CA (1981) Oxidation-reduction physical chemistry of the acceptor quinone complex in bacterial photosynthetic reaction centers: evidence for a new model of herbicide activity. Isr J Chem 21: 348–354.
Wraight, CA (1982) Current attitudes in photosynthesis research. In: Govindjee (ed.) Photosynthesis, Volume I, pp 17–61. New York: Academic Press.
Wraight, CA (1985) Modulation of herbicide-binding by the redox state of Q400, an endogenous component of photosystem II. Biochim Biophys Acta 809: 320–330.
Wurmser, R (1987) Letter to the editor. Photosynth Res 13: 91–93.
Wydrzynski, T and Govindjee (1975) A new site of bicarbonate effect in photosystem II of photosynthesis: evidence from chlorophyll fluorescence transients in spinach chloroplasts. Biochim Biophys Acta 387: 403–408.
Yoshida, T, Morita, N, Tamiya, H, Nakayama, H and Huzisige, H (1924) Über den Gehalt des Assimilationssauerstoffs an schwerem Isotop. Ein Beitrag zur kenntnis des Mechanismus der Photosynthese. Acta Phytochim 13: 11–18.
Zilinskas BA (1975) Photosystem II reactions in thylakoid membranes. Ph.D. Thesis, University of Illinois at Urbana-Champaign.
Zillinskas, B and Govindjee (1975) Silicomolybdate and silicotungstate mediated dichlorophenyldimethylurea-insensitive photosystem II reaction: electron flow, chlorophyll a fluorescence and delayed light emission changes. Biochim Biophys Acta 387: 306–319.
Zimmermann, J-L and Rutherford, AW (1986) Photoreductant-induced oxidation of Fe2+ in the electron-acceptor complex of photosystem II. Biochim Biophys Acta 851: 416–423.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Blubaugh, D.J., Govindjee The molecular mechanism of the bicarbonate effect at the plastoquinone reductase site of photosynthesis. Photosynth Res 19, 85–128 (1988). https://doi.org/10.1007/BF00114571
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00114571