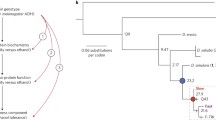
Abstract
Evolutionary genetics embodies a broad research area that ranges from the DNA level to studies of genetic aspects in populations. In all cases the purpose is to determine the impact of genetic variation on evolutionary change. The broad range of evolutionary genetics requires the involvement of a diverse group of researchers: molecular biologists, (population) geneticists, biochemists, physiologists, ecologists, ethologists and theorists, each of which has its own insights and interests. For example, biochemists are often not concerned with the physiological function of a protein (with respect to pH, substrates, temperature, etc.), while ecologists, in turn, are often not interested in the biochemical-physiological aspects underlying the traits they study. This review deals with several evolutionary aspects of the Drosophila alcohol dehydrogenase gene-enzyme system, and includes my own personal viewpoints. I have tried to condense and integrate the current knowledge in this field as it has developed since the comprehensive review by van Delden (1982). Details on specific issues may be gained from Sofer and Martin (1987), Sullivan, Atkinson and Starmer (1990); Chambers (1988, 1991); Geer, Miller and Heinstra (1991); and Winberg and McKinley-McKee (1992).
Similar content being viewed by others
References
AndersonS. M. & J. F.McDonald, 1981. Effect of environmental alcohol on in vivo properties of Drosophila alcohol dehydrogenase. Biochem. Genet. 19: 421–430.
AndersonS. M. & J. F.McDonald, 1983. Biochemical and molecular analysis of naturally occurring Adh variants in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 80: 4798–4802.
AndersonS. M. & S. E.Barnett, 1991. The involvement of alcohol and aldehyde dehydrogenase in alcohol/aldehyde metabolism in Drosophila melanogaster. Genetica 83: 99–106.
AndersonS. M., M. R.Brown & J. F.McDonald, 1991. Tissue specific expression of the Drosophila Adh-gene: a comparison of in situ hybridization and immunocytochemistry. Genetica 84: 95–100.
AquadroC. F., S. F.Desse, M. M.Bland, C. H.Langley & C. C.Laurie-Ahlberg, 1986. Molecular population genetics of the alcohol dehydrogenase gene region of Drosophila melanogaster. Genetics 114: 1165–1190.
AquadroC. F., 1992. Why is the genome variable? Insights from Drosophila. Trends in Genet. 8: 355–362.
AshburnerM., M.Bodmer & F.Lemeunier, 1984. On the evolutionary relationships of Drosophila melanogaster. Devel. Genet. 4: 295–312.
Belfort, M., 1991. Self-splicing introns in prokaryotes: Migrant fossils? Cell: 9–11.
BennerS. A. & A. DElington, 1988. Interpreting the behavior of enzymes, purpose or pedigree? Crit. Rev. Biochem. 23: 369–426.
BenyajatiC., A. R.Place, D. A.Powers & W.Sofer, 1981. Alcohol dehydrogenase gene of Drosophila melanogaster: relationship of intervening sequences to functional domains in the protein. Proc. Natl. Acad. Sci. USA 78: 2717–2721.
Bijlsma-MeelisE., 1979. Viability in Drosophila melanogaster in relation to age and ADH activity of eggs transferred to ethanol food. Heredity 42: 79–89.
Bijlsma-MeelisE. & R.Bijlsma, 1988. The alcohol dehydrogenase polymorphism in Drosophila melanogaster: fitness measurements and predictions under conditions with no alcohol stress. Genetics 120: 743–753.
BodmerM. & M.Ashburner, 1984. Conservation and change in the DNA sequence coding for alcohol dehydrogenase in sibling species of Drosophila. Nature 309: 425–430.
BradyJ. P. & R. C.Richmond, 1992. An evolutionary model for duplication and divergence of esterase genes in Drosophila. J. Mol. Evol. 34: 506–521.
BriscoeD. A., A.Robertson & J.Malpica, 1975. Dominance at Adh locus in response of adult Drosophila melanogaster to environmental alcohol. Nature 255: 148–149.
CacconeA. & J.R.Powell, 1990. Extreme rates and heterogeneity in insect DNA evolution. J. Mol. Evol. 30: 273–280.
CavenerD.R. & M.Clegg, 1981a. Evidence for biochemical and physiological differences between enzyme genotypes in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 78: 4444–4447.
CavenerD. R. & M.Clegg, 1981b. Multigenic response to ethanol in Drosophila melanogaster. Evolution 35: 1–10.
ChambersG. K., 1988. The Drosophila alcohol dehydrogenase gene-enzyme system. Adv. Genet. 25: 40–107.
ChambersG. K., 1991. Gene expression, adaptation and evolution in higher organisms. Evidence from studies of Drosophila alcohol dehydrogenases. Comp. Biochem. Physiol. 99B: 723–730.
ChenZ., L.Lu, W.Lee & S. H.Chang, 1991. Role of aspartic acid 38 in the cofactor specificity of Drosophila alcohol dehydrogenase. Eur. J. Biochem. 202: 263–267.
ChoudharyM. & C. C.Laurie, 1991. Use of in vitro mutagenesis to analyze the molecular basis of the difference in Adh expression associated with the aliozyme polymorphism in Drosophila melanogaster. Genetics 129: 481–488.
ClarkA. G. & L. E.Keith, 1988. Variation among extracted lines of Drosophila melanogaster in triacylglycerol and carbohydrate storage. Genetics 119: 595–607.
ClarkA. G., 1990. Genetic components of variation in energy storage in Drosophila melanogaster. Evolution 44: 637–650.
ClarkA. G., 1991. Mutation-selection balance and metabolic control theory. Genetics 129: 909–923.
ClarkeB., 1975. The contribution of ecological genetics to evolutionary theory: detecting the direct effects of natural selection on particular polymorphic loci. Genetics 79a: 101–113.
CohanF. M. & A. A.Hoffmann, 1986. Genetic divergence under uniform selection. II. Different responses to selection for knockdown resistance to ethanol among Drosophila melanogaster populations and their replicate lines. Genetics 114: 145–163.
ColletC., 1988. Recent origin for a thermostable alcohol dehydrogenase allele of Drosophila melanogaster. J. Mol. Evol. 27: 142–146.
DavidJ. R. & C.Bocquet, 1976. Compared toxicities of different alcohols for two Drosophila sibling species: D. melanogaster and D. simulans. Comp. Biochem. Physiol. 54C: 71–74.
DavidJ. R., C.Bocquet, J.vanHerrewege, P.Fouillet & M.Arens, 1978. Alcohol metabolism in Drosophila melanogaster: Uselessness of the most active aldehyde oxidase produced by the Aldox-locus. Biochem. Genet. 16: 203–211.
DavidJ. R. & J.vanHerrewege 1983. Adaptation to alcoholic fermentation in Drosophila species: relationship between alcohol tolerance and larval habitat. Comp. Biochem. Physiol. 74A: 283–288.
DavidJ. R., 1988. Ethanol adaptation and alcohol dehydrogenase polymorphism in Drosophila: from phenotypic functions to genetic structures. In: Population Genetics and Evolution (G.deJong, ed.) pp. 163–172. Springer Verlag, Berlin.
DayT. H., P. C.Hillier & B.Clarke, 1974. Properties of genetically polymorphic isozymes of alcohol dehydrogenase in Drosophila melanogaster. Biochem. Genet. 11: 141–153.
DeJongG. & W.Scharloo, 1976. Environmental determination of selective significance or neutrality of amylase variants in Drosophila melanogaster. Genetics 84: 77–94.
Deltombe-LietaertM. C., J.Deleour, N.Lenelle-Montfort & A.Elens, 1979. Ethanol metabolism in Drosophila melanogaster. Experientia 35: 579–581.
DickinsonW. J., R. G.Rowan & M. D.Brennan, 1984. Regulatory gene evolution: adaptive differences in expression of alcohol dehydrogenase in Drosophila melanogaster and D. simulans. Heredity 52: 215–225.
DuesterG., H.Jörnvall & G. W.Hatfield, 1986. Intron-dependent evolution of the nucleotide-binding domains within alcohol dehydrogenase and related enzymes. Nucleic Acids Res. 14: 1931–1941.
DykhuizenD. E., A. M.Dean & D. L.Hartl, 1987. Metabolic flux and fitness. Genetics 115: 25–31.
DykhuizenD. E. & A. M.Dean, 1990. Enzyme activity and fitness: evolution in solution. Trends Ecol. Evol. 5: 257–262.
EissesK. Th., W. G. E. J.Schoonen, W.Aben, W.Scharloo & G. E. W.Thörig, 1985. Dual function of the alcohol dehydrogenase of Drosophila melanogaster: ethanol and acetaldehyde oxidation by two alleloenzymes ADH-71k and ADH-F. Mol. Gen. Genet. 199: 76–81.
EissesK. Th., 1989. On the oxidation of aldehydes by alcohol dehydrogenase of Drosophila melanogaster: evidence for the gem-diol as the reacting substrate. Bioorg. Chem. 17: 268–274.
EissesK. Th., A. J.Andriesse, A. D.deBoer, G. E. W.Thörig & P. J.Weisbeek, 1990. Analysis of the gene encoding the multifunctional alcohol dehydrogenase ADH-71k of Drosophila melanogaster. Mol. Biol. Evol. 7: 459–469.
EtgesW. J. & C. S.Klassen, 1989. Influences of atmospheric ethanol on adult Drosophila mojavensis: altered metabolic rates and increases in fitness among populations. Physiol. Zool. 62: 170–193.
FellD. A., 1992. Metabolic control analysis: a survey of its theoretical and experimental development. Biochem. J. 286: 313–330.
FibiaJ., L.Enjuanes & R.Gonzàlez-Duarte, 1989. Inter-specific analysis of Drosophila alcohol dehydrogenase by an immunoenzymatic assay using monoclonal antibodies. Biochem. Biophys. Res. Commun. 160: 638–646.
FibliaJ., S.Atrian & R.Gonzàlez-Duarte, 1993. Evidence of serine-protease activity closely associated with drosophila alcohol dehydrogenase. Eur. J. Biochem. 211: 357–365.
FershtA. R., 1985. Enzyme Structure and Mechanism. Second edition, Freeman & Company, New York.
FreriksenA., D.Seykens, W.Scharloo & P. W. H.Heinstra, 1991. Alcohol dehydrogenase controls the flux from ethanol into lipids in Drosophila larvae: a 13C NMR study. J. Biol. Chem. 266: 21399–21403.
Freriksen, A., D. Seykens & P. W. H. Heinstra, 1993. Differences between larval and adult Drosophila in metabolic degradation of ethanol. Evolution, in press.
Freriksen, A., B. L. A. de Ruiter, H-J. Groenenberg, W. Scharloo & P. W. H. Heinstra, 1993a. Multilevel approach to the significance of genetic variation in alcohol dehydrogenase of Drosophila. Evolution, in press.
Freriksen, A., B. L. A. de Ruiter, W. Scharloo & P. W. H. Heinstra, 1993b. Drosophila alcohol dehydrogenase polymorphism and carbon-13 fluxes: opportunities for epistasis and natural selection. Genetics, submitted.
FreriksenA. & P. W. H.Heinstra, 1993. A novel ancestral protein of Drosophila alcohol dehydrogenase in Streptomyces? Biochem. Genet. 31: 391–405.
GarcinF. J., J.Côté, S.Radouco-Thomas, D.Kasenczuk, S.Chawla & C.Radouco-Thomas, 1983. Acetaldehyde oxidation in Drosophila melanogaster and D. simulans: evidence for the presence of an NAD+-dependent aldehyde dehydrogenase. Comp. Biochem. Physiol. 75B: 205–210.
GarcinF. J., HinG. L., CôtéJ., Radouco-ThomasC., ChawlaS. & Radouco-ThomasC., 1985. Aldehyde dehydrogenase in Drosophila: developmental and functional aspects. Alcohol 2: 85–89.
GeerB. W., S. W.McKechnie & M. L.Langevin, 1983. Regulation of sn-glycerol-3-phosphate dehydrogenase in Drosophila melanogaster larvae by dictary ethanol and sucrose. J. Nutr. 113: 1632–1642.
GeerB. W., M. L.Langevin & S. W.McKechnie, 1985. Dictary ethanol and lipid synthesis in Drosophila melanogaster. Biochem. Genet. 23: 607–622.
GeerB. W., S. W.McKechnie, M. M.Bentley, J. G.Oakeshott, E. M.Quinn & M. L.Langevin, 1988. Induction of alcohol dehydrogenase by ethanol in Drosophila melanogaster. J. Nutr. 118: 398–407.
GeerB. W., L. K.Dybas & L. J.Shanner, 1989. Alcohol dehydrogenase and ethanol tolerance at the cellular level in Drosophila melanogaster. J. Exp. Zool. 250: 22–39.
GeerB. W., P. W. H.Heinstra, A. M.Kapoun & A.van derZel, 1990. Alcohol dehydrogenase and alcohol tolerance in Drosophila melanogaster. In: Ecological and Evolutionary Genetics of Drosophila (J. S. F.Barker et al., eds) pp. 231–252, Plenum Press, New York.
GeerB. W., R. R.MillerJr & P. W. H.Heinstra, 1991. Genetic and dictary control of alcohol degradation in Drosophila. In: Drug and Alcohol Abuse Reviews (R. R.Watson, ed.). Vol. 2 pp. 325–373. The Humana Press Inc. Clifton, New Jersey.
GeerB. W., S. W.McKechnie, P. W. H.Heinstra & M.Pyka, 1991. Heritable variation in ethanol tolerance and its association with biochemical traits in Drosophila melanogaster. Evolution 45: 1107–1119.
GeerB. W., P. W. H.Heinstra & S. W.McKechnie, 1993. The biological basis of ethanol tolerance in Drosophila. Comp. Biochem. Physiol. 105B: 203–229.
GelfandL. J. & J. F.McDonald, 1980. Relationship between ADH activity and behavioral response to environmental alcohol in Drosophila. Behav. Genet. 10: 237–249.
GibsonJ. B., 1972. Differences in the number of molecules produced by two allelic electrophoretic enzyme variants in Drosophila melanogaster. Experientia 28: 975–976.
GibsonJ. B., T. W.May & A. V.Wilks, 1981. Genetic variation at the alcohol dehydrogenase locus in Drosophila melanogaster in relation to environmental variation: ethanol levels in breeding sites and allozyme frequencies. Oecologia 51: 191–198.
GibsonJ. B. & J. G.Oakeshott, 1982. Tests of the adaptive significance of the alcohol dehydrogenase polymorphism in Drosophila melanogaster: paths, pitfalls and prospects. In: Ecological Genetics and Evolution (J. S. F.Barker & W. T.Starmer, eds.) pp. 291–306. Academic Press, Australia.
GibsonJ. B. & A. V.Wilks, 1988. The alcohol dehydrogenase polymorphism of Drosophila melanogaster in relation to environmental ethanol, ethanol tolerance and alcohol dehydrogenase activity. Heredity 60: 403–414.
GillespieJ. H., 1991. The causes of molecular evolution. Oxford University Press. New York, Oxford.
HagemanJ., K. Th.Eisses, P. J. M.Jacobs & W.Scharloo, 1990. Ethanol in Drosophila cultures as a selective factor. Evolution 44: 447–454.
HartlD. L. & D. E.Dykhuizen, 1981. Potential for selection among nearly neutral allozymes of 6-phosphogluconate dehydrogenase in Escherichia coli. Proc. Natl. Acad. Sci. USA 78: 6344–6348.
HartlD. L., D. E.Dykhuizen & A. M.Dean, 1985. Limits of adaptation: the evolution of selective neutrality. Genetics 111: 655–674.
Hartman, J. R., L. K. Dybas & B. W. Geer, 1993. At high dietary levels ethanol alters the structure of mid- and hindgut cells of Drosophila melanogaster larvae. J. Exp. Zool., in press.
HeinstraP. W. H., K. Th.Eisses, W. G. E. J.Schoonen, W. J. M.Aben, A. J.deWinter, D. J.van derHorst, W. J.vanMarrewijk, A. M. Th.Beenakkers, W.Scharloo & G. E. W.Thörig, 1986. A dual function of alcohol dehydrogenase in Drosophila. Genetica 60: 129–137.
HeinstraP. W. H., W. J. M.Aben, W.Scharloo & G. E. W.Thörig, 1986. Alcohol dehydrogenase of Drosophila melanogaster: metabolic differences mediated through cryptic allozymes. Heredity 57: 23–29.
HeinstraP. W. H., W.Scharloo & G. E. W.Thörig, 1987. Physiological significance of the alcohol dehydrogenase polymorphism in larvae of Drosophila. Genetics 117: 75–84
HeinstraP. W. H., W.Scharloo & G. E. W.Thörig, 1988. Alcohol dehydrogenase polymorphism in Drosophila: enzyme kinetics of product inhibition. J. Mol. Evol. 28: 145–150.
HeinstraP. W. H., G. E. W.Thörig, W.Scharloo, W.Drenth & R. J. M.Nolte, 1988. Kinetics and thermodynamics of ethanol oxidation catalyzed by genetic variants of the alcohol dehydrogenase from Drosophila melanogaster and D. simulans. Biochim. Biophys. Acta 967: 224–233.
HeinstraP. W. H., B. W.Geer, D.Seykens & M. L.Langevin, 1989. The metabolism of ethanol-derived acetaldehyde by alcohol dehydrogenase (EC 1.1.1.1) and aldehyde dehydrogenase (EC 1.2.1.3) in Drosophila melanogaster larvae. Biochem. J. 259: 791–797.
HeinstraP. W. H., D.Seykens, A.Freriksen & B. W.Geer, 1990. Metabolic-physiology of alcohol degradation and adaptation in Drosophila larvae as studied by means of carbon-13 nuclear magnetic resonance spectroscopy. Insect Biochem. 20: 343–348.
HeinstraP. W. H. & B. W.Geer, 1991. Metabolic control analysis and enzyme variation: nutritional manipulation of the flux from ethanol to lipids in Drosophila. Mol. Biol. Evol. 8: 703–708.
Heinstra, P. W. H., M. E. Whiteside, J. Ochoa-Nelson, C. Baumgardner, C. C. Laurie & B. W. Geer, 1993. The flux control coefficient for alcohol dehydrogenase varies over a range of activity due to Adh gene dosage modification in Drosophila melanogaster. In preparation.
HoffmannA. A. & S. W.McKechnie, 1991. Heritable variation in resource utilization and response in a winery population of Drosophila melanogaster. Evolution 45: 1000–1015.
JeffsP. & M.Ashburner, 1991. Processed pseudogenes in Drosophila. Proc. R. Soc. Lond. 244: 151–159.
JiangJ. C., J. B.Gibson & H.Chen, 1989. Genetic differentiation in populations of Drosophila melanogaster from the People's Republic of China: comparison with patterns on other continents. Heredity 62: 193–198.
JiangJ. C. & J. B.Gibson, 1992. The alcohol dehydrogenase polymorphism in natural populations of Drosophila melanogaster: ADH activity variation, restriction site polymorphism and the Adh cline. Heredity 68: 337–344.
JörnvallH., B.Persson, M.Krook & R.Kaiser, 1990. Alcohol dehydrogenases. Biochem. Soc. Trans. 18: 169–171.
KapounA. M., B. W.Geer, P. W. H.Heinstra, V.Corbin & S. W.McKechnie, 1990. Molecular control of the induction of alcohol dehydrogenase by ethanol in Drosophila melanogaster larvae. Genetics 124: 881–888.
KeightleyP. D. & H.Kacser, 1987. Dominance, pleiotropy and metabolite structure. Genetics 117: 319–329.
KerverW. J. M. & G.Rotman, 1987. Development of ethanol tolerance in relation to the alcohol dehydrogenase locus in Drosophila melanogaster. II. The influence of phenotypic adaptation and maternal effect on survival on alcohol supplemented media. Heredity 58: 239–248.
KimuraM., 1983. The neutral theory of molecular evolution. Cambridge University Press, Cambridge, UK.
KreitmanM., 1983. Nucleotide polymorphism at the alcohol dehydrogenase locus of Drosophila melanogaster. Nature 304: 412–417.
KreitmanM., 1987. Molecular population genetics. In: Oxford surveys in evolutionary biology (P. H.Harvey & L.Partridge, eds) pp. 38–60, Oxford University Press, Oxford, UK.
KreitmanM. & R. R.Hudson, 1991. Inferring the evolutionary histories of the Adh and Adh-dup loci in Drosophila melanogaster from patterns of polymorphism and divergence. Genetics 127: 565–582.
KrozowskiZ., 1992. 11β-Hydroxysteroid dehydrogenase and the short-chain alcohol dehydrogenase (SCAD) superfamily. Mol. Cell. Endocrinol. 84C: 25–31.
LaurieC. C. & L. F.Stam, 1988. Quantitative analysis of RNA produced by Slow and Fast alleles of Adh in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 85: 5161–5165.
LaurieC. C., E. M.Heath, J. W.Jacobson & M. S.Thomson, 1990. Genetic basis of the difference in alcohol dehydrogenase expression between Drosophila melanogaster and D. simulans. Proc. Natl. Acad. Sci. USA 87: 9674–9678.
LaurieC. C., J. T.Bridgham & M.Choudhary, 1991. Associations between DNA sequence variation and variation in expression of the Adh gene in natural populations of Drosophila melanogaster. Genetics 129: 489–499.
LealJ. F. & M.Barbancho, 1992. A etaldehyde detoxification mechanisms in Drosophila melanogaster adults involving aldehyde dehydrogenase (ALDH) and alcohol dehydrogenase (ADH) enzymes. Insect Biochem. Molec. Biol. 22: 885–892.
LealJ. F. & M.Barbancho, 1993. Aldehyde dehydrogenase (ALDH) activity in Drosophila melanogaster adults: evidence for cytosolic localization. Insect Biochem. Molec. Biol. 23: 543–547.
LewisN. & J. B.Gibson, 1978. Variation in amount of enzyme protein in natural populations. Biochem. Genet. 16: 159–170.
LissemoreJ. L., C. A.Baumgardner, B. W.Geer & D. T.Sullivan, 1990. Effect of dietary carbohydrates and ethanol on expression of genes encoding glycerol-3-phosphate dehydrogenase, aldolase, and phosphoglycerate kinase in Drosophila larvae. Biochem. Genet. 28: 615–630.
LongM. & C. H.Langley, 1993. Natural selection and the origin of jingwei, a chimeric processed functional gene in Drosophila. Science 260: 91–95.
MarfanyG. & R.Gonzàlez-Duarte, 1991. The Adh genomic region of Drosophila ambigua: evolutionary trends in different species. J. Mol. Evol. 32: 454–462.
MarfanyG & R.Gonzàlez-Duarte, 1992. The Drosophila subobseura Adh genomic region contains valuable evolutionary markers. Mol. Biol. Evol. 9: 261–277.
MatthewP., A.Agrotis, C.Taylor & S. W.McKechnie, 1992. An association between ADH protein levels and polymorphic nucleotide variation in the Adh gene of Drosophila melanogaster. Mol. Biol. Evol. 9: 526–536.
McDonaldJ. F., S. M.Anderson & M.Santos, 1980. Biochemical differences between products of the Adh locus in Drosophila. Genetics 95: 1013–1022.
McDonaldJ. H. & M.Kreitman, 1991. Adaptive protein evolution at the Adh locus in Drosophila. Nature 351: 652–654.
McKechnieS. W. & P.Morgan, 1982. Alcohol dehydrogenase polymorphism of Drosophila melanogaster: aspects of alcohol and temperature variation in the larval environment. Aust. J. Biol. Sci. 95: 85–93.
McKechnieS. W. & J. A.McKenzie, 1983. Polymorphism of alcohol dehydrogenase (ADH) in a winery cellar population of Drosophila melanogaster: gene frequency association with temperature and genotype differences in progeny production. Evolution 37: 850–853.
McKechnieS. W. & B. W.Geer, 1984. Regulation of alcohol dehydrogenase in Drosophila melanogaster by dietary alcohol and carbohydrate. Insect Biochem. 14: 231–242.
McKechnieS. W. & B. W.Geer, 1988. The epistasis of Adh and Gpdh allozymes and variation in the ethanol tolerance of Drosophila melanogaster larvae. Genet. Res. Camb. 52: 179–184.
McKechnieS. W., J. L.Ross & K. L.Turney, 1990. Environmental modulation of sn-glycerol-3-phosphate oxidase (GPO) activity in larvae of Drosophila melanogaster. In: Ecological and Evolutionary Genetics of Drosophila (J. S. F.Barker et al., eds.) pp. 253–271, Plenum Press, New York.
McKenzieJ. A. & P. A.Parsons, 1972. Alcohol tolerance: an ecological parameter in the relative success of Drosophila melanogaster and D. simulans. Oecologia 10: 373–388.
McKenzieJ. A. & P. A.Parsons, 1974. Microdifferentiation in a natural population of Drosophila melanogaster to alcohol in the environment. Genetics 77: 385–394.
McKenzieJ. A. & S. W.McKechnie, 1979. A comparative study of resource utilization in natural populations of Drosophila melanogaster and D. simulans. Oecologia 40: 299–309.
MiddletonR & H.Kacser, 1983. Enzyme variation, metabolic flux, and fitness: alcohol dehydrogenase in Drosophila melanogaster. Genetics 105: 633–650.
MillerR. R.Jr., P. W. H.Heinstra & B. W.Geer, 1992. Metabolic flux and the role of lipid in alcohol tolerance in Drosophila. In: Nutrition & Alcohol (R. R.Watson & B.Watzl, eds) pp. 205–244, CRC Press, Boca Raton, FL, USA.
MoxonL. N., R. S.Holmes, P. A.Parsons, M. G.Irving & D. M.Doddrell, 1985. Purification and molecular properties of alcohol dehydrogenase from Drosophila melanogaster: evidence from NMR and kinetic studies for function as an aldehyde dehydrogenase. Comp. Biochem. Physiol. 80B: 525–535.
NeidleE., C.Hartnett, L. N.Ornston, S. A.Bairoch, M.Rekik & S.Harayama, 1992. Cis-diol dehydrogenases encoded by the TOLpWWO plasmid xylL gene and the Acinetobacter calcoaceticus chromosomal benD gene are members of the short-chain alcohol dehydrogenase superfamily. Eur. J. Biochem. 204: 113–120.
OakeshottJ. G., 1976. Selection at the alcohol dehydrogenase locus in Drosophila melanogaster imposed by environmental ethanol. Genet. Res. Camb. 26: 265–274.
OakeshottJ. G., J. B.Gibson, P. R.Anderson, W. R.Knibb, D. G.Anderson & G. K.Chambers, 1982. Alcohol dehydrogenase and glycerol-3-phosphate dehydrogenase clines in Drosophila melanogaster on different continents. Evolution 36: 86–96.
OlliverS. G., Q. J. M.van derAart, M. L.Agostoni-Carbone, M. L., et al., 1992. The complete DNA sequence of yeast chromosome III. Nature 357: 38–46.
OudmanL., W.vanDelden, A.Kamping & R.Bijlsma, 1991. Polymorphism at the Adh and α-Gpdh loci in Drosophila melanogaster: effects of rearing temperature on developmental rate, body weight, and some blochemical parameters. Heredity 67: 103–115.
OudmanL., W.vanDelden, A.Kamping & R.Bijlsma, 1992. Interaction between the Adh and α-Gpdh loci in Drosophila melanogaster: adult survival at high temperature. Heredity 68: 289–297.
Pecsenye, K., 1990. Ethanol stress in Drosophila melanogaster. Ph.D. thesis. University of Umeå, Sweden.
PerssonB., M.Krook & H.Jörnvall, 1991. Characteristics of short-chain alcohol dehydrogenases and related enzymes. Eur. J. Biochem. 200: 537–543.
PowersD. A., T.Lauerman, D.Crawford & L.DiMichelle, 1991. Genetic mechanisms for adapting to a changing environment. Ann. Rev. Genet. 25: 629–659.
RatL., M.Veuille & J. A.Lepesant, 1991. Drosophila fat body protein P6 and alcohol dehydrogenase are derived from a common ancestral protein. J. Mol. Evol. 33: 194–203.
ReyM., A. M.Palermo & E. R.Muñoz, 1992. Nondisjuction induced by ethanol in Drosophila melanogaster females. Mutation Res. 268: 95–104.
RogersJ. H., 1990. The role of introns in evolution. FEBS Lett. 268: 339–343.
SavakisC., M.Ashburner & J. H.Willis, 1986. The expression of the gene coding for alcohol dehydrogenase during the development of Drosophila melanogaster. Devel. Biol. 114: 194–207.
ScharlooW., 1988. Selection on morphological patterns. In: Population Genetics and Evolution (G.deJong, ed.) pp. 230–250, Springer Verlag, Berlin.
SchaefferS. W. & C. F.Aquadro, 1987. Nucleotide sequence of the Adh gene region of Drosophila pseudoobscura: evolutionary changes and evidence for an ancient gene duplication. Genetics 117: 61–73.
SchaefferS. W. & E. L.Miller, 1992. Molecular population genetics of an electrophoretically monomorphic protein in the alcohol dehydrogenase region of Drosophila pseudoobscura. Genetics 132: 163–178.
SimmonsG. M., M.Kreitman, W. F.Quattlebaum & N.Miyashita, 1989. Molecular analysis of the alleles of alcohol dehydrogenase along a cline in Drosophila melanogaster. I. Maine, North Carolina, and Florida. Evolution 43: 393–409.
SoferW. & P. F.Martin, 1987. Analysis of alcohol dehydrogenase gene expression in Drosophila. Annu. Rev. Genet. 21: 203–235.
StarmerW. T. & D. T.Sullivan, 1989. A shift in third-codon position nucleotide frequency in alcohol dehydrogenase genes in the genus Drosophila. Mol. Biol. Evol. 6: 546–552.
StephensJ. C. & M.Nei, 1985. Phylogenetic analysis of polymorphic DNA sequences at the Adh locus in Drosophila melanogaster and its sibling species. J. Mol. Evol. 22: 289–300.
SullivanD. T., P. W.Atkinson & W. T.Starmer, 1990. Molecular evolution of the alcohol dehydrogenase genes in the genus Drosophila. In: Evolutionary Biology (M.Hecht, B.Wallace & G.Prance, eds.) Vol. 24 pp. 107–147. Plenum Press London and New York.
ThatcherD. R., 1980. The complete amino acid sequence of three alcohol dehydrogenase alleloenzymes from the fruit fly Drosophila. Biochem. J. 187; 875–883.
ThomsonM. S., J. W.Jacobson & C. C.Laurie, 1991. Comparison of alcohol dehydrogenase expression in Drosophila melanogaster and D. simulans. Mol. Biol. Evol. 8: 31–48.
vanDeldenW., 1982. The alcohol dehydrogenase polymorphism in Drosophila melanogaster: selection at an enzyme locus. In: Evolutionary Biology (M.Hecht, B.Wallace & G.Prance, eds.) Vol. 15. pp. 187–222. Plenum Press London and New York
vanDeldenW., A. C.Boerema & A.Kamping, 1978. The alcohol dehydrogenase polymorphism in populations of Drosophila melanogaster. I. Selection in different environments. Genetics 90: 161–191.
vanDeldenW. & A.Kamping, 1979. The alcohol dehydrogenase polymorphism in populations of Drosophila melanogaster. 3. Differences in developmental time. Genet. Res. Camb. 33: 15–27.
vanDeldenW. & A.Kamping, 1988. Selection against Adh null alleles in Drosophila melanogaster. Heredity 61: 209–216.
van derZelA., R.Dadoo, B. W.Geer & P. W. H.Heinstra, 1990. The involvement of catalase in alcohol metabolism in Drosophila melanogaster larvae. Arch. Biochem. Biophys. 287: 121–127.
VillarroyaA. & E.Juan, 1991. ADH and phylogenetic relationships of Drosophila lebanonensis (Scaptodrosophila). J. Mol. Evol. 32: 421–428.
Vouidibio, J., P. Capy, D. Defaye, E. Pla, J. Sandrin, A. Csink & J. R. David, 1989. Short-range genetic structure of Drosophila melanogaster populations in an Afrotropical urban area and its significance. Proc. Natl. Acad. Sci. USA 8442-8446.
WalshK. & D. E.Koshland, 1985. Characterization of ratecontrolling steps in vivo by use of an adjustable expression vector. Proc. Natl. Acad. Sci. USA 82: 3577–3581.
WattW. B., 1985. Bioenergetics and evolutionary genetics: opportunities for new synthesis. Am. Nat. 125: 118–143.
WinbergJ-O., R.Hovik & J. S.McKinley-McKee, 1985. The alcohol dehydrogenase alleloenzymes ADH-S and ADH-F from the fruit fly Drosophila melanogaster: an enzymatic rate assay to determine the active-site concentration. Biochem. Genet. 23: 205–216.
WinbergJ-O. & J. S.McKinley-McKee, 1992. Kinetic interpretations of active site topologies and residue exchanges in Drosophila alcohol dehydrogenases. Int. J. Biochem. 24: 169–181.
ZeraA. J., R. K.Koehn & J. G.Hall, 1985. Allozymes and biochemical adaptation. In: Comprehensive insect physiology, biochemistry, and pharmacology (G. A.Kerkut & L. I.Gilbert, eds.) Vol. 10. pp. 633–674. Pergamon Press New York.
Author information
Authors and Affiliations
Additional information
Dedicated to Professor Billy W. Geer, because of his contributions to knowledge of the biochemical genetics of Drosophila.
Rights and permissions
About this article
Cite this article
Heinstra, P.W.H. Evolutionary genetics of the Drosophila alcohol dehydrogenase gene-enzyme system. Genetica 92, 1–22 (1993). https://doi.org/10.1007/BF00057503
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00057503