Summary
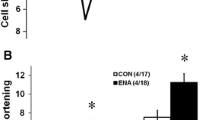
Recent studies have suggested the beneficial effects of angiotensin converting enzyme (ACE) inhibitors against myocardial ischemic-reperfusion injury. This study was designed to compare the cardioprotective effects of two sulfhydryl ACE inhibitors, captopril and zofenopril, with those of a nonsulfhydryl ACE inhibitor, fosinopril. The efficacy of these ACE inhibitors to scavenge oxygen radicals in vitro were also examined. Isolated rat hearts perfused by the Langendorff technique were preperfused in the presence or absence of ACE inhibitors (50 μm for 15 minutes), and the hearts were then subjected to 30 minutes of ischemia followed by 30 minutes of reperfusion. Zofenopril and captopril, but not fosinopril, improved postischemic left ventricular functions and reduced myocardial cellular injury, as evidenced by improved recovery of the first derivative of left ventricular pressure development and reduced creatine kinase release compared with control (p<.05). Coronary flow was significantly increased by captopril and zofenopril only. The same two drugs also inhibited the enhanced lipid peroxidation during reperfusion. Although significant differences were not noticed in the postischemic myocardial membrane phospholipid composition, captopril and zofenopril reduced nonesterified fatty acid contents, including palmitic, linoleic, oleic, and arachidonic acids. In vitro studies demonstrated that captopril and zofenopril were able to scavenge hydroxyl radicals. These results indicate that among three ACE inhibitors, two sulfhydryl-containing drugs, captopril and zofenopril, possess cardioprotective as well as free-radical scavenging abilites. Attenuation of phospholipid degradation and lipid peroxidation may be contributory to the protective effects observed in this study.
Similar content being viewed by others
References
Ellis SG, Henschke CI, Sandor T, et al. Time course of functional and biochemical recovery of myocardium salvaged by reperfusion. J Am Coll Cardiol 1984;1:1047–1055.
Przyklenk K, Vivaldi MT, Schoen T-J, et al. Salvage of ischemic myocardium by reperfusion: Importance of collateral blood flow and myocardial oxygen demand during occlusion. Cardiovasc Res 1986;20:403–414.
Wijins W, Serrays PW, Slager CJ, et al. Effect of coronary occlusion during percutaneous transluminal angioplasty in humans on left ventricular chamber stiffness and regional diastolic pressure-radius relations. J Am Coll Cardiol 1986;7:455–463.
Przyklenk K, Kloner RA. Relationships between structure and effects of ACE inhibitors: Comparative effects in myocardial ischemic/reperfusion injury. Br J Clin Pharmacol 1989;28:167S-175S.
Braunwald E. The aggressive treatment of acute myocardial infarction. Circulation 1985;71:1087–1092.
Billi R. Oxygen-derived free radicals and postischemic myocardial dysfunction (stunned myocardium). J Am Coll Cardiol 1988;12:239–249.
Li K, Chen X. Protective effects of captopril and enalapril on myocardial ischemia and reperfusion damage of rat. J Mol Cell Cardiol 1987;19:909–915.
Ertl G, Kloner RA, Alexander RW, Braunwald E. Limitation of experimental infarct size by an angiotensin-converting enzyme inhibitor. Circulation 1982;65:40–48.
Westline W, Mullane K. Does captopril attenuate reperfusion-induced myocardial dysfunction by scavenging free radicals? Circulation 1988;77 (Suppl I):I30-I39.
van Gilst WH, de Graeff PA, Wesseling H, de Langen CDJ. Reduction of reperfusion arrhythmias in the ischemic isolated rat heart by angiotensin-converting enzyme inhibitors: A comparison of captopril, enalapril, and HOE498. J Cardiovasc Pharmacol 1986;8:722–728.
Otani H, Prasad RM, Das DK. Mechanism of membrane phospholipid degradation in ischemic-reperfused rat heart. Am J Physiol 1989;257:H252-H258.
Folch J, Less M, Sloan-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissue. J Biol Chem 1957;226:497–509.
Gilfillan AM, Chu AJ, Smart DA, Rooney SA. Single plate separation of lung phospholipids including disaturated phosphatidylcholine. J Lipid Res 1983;24:1651–1656.
Kaluzny MA, Duncan CA, Merrit MV, Epps DE. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J Lipid Res 1985;26:135–140.
Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem 1959;234:466–468.
Prasad MR, Jones RM, Young HS, et al. Analysis of tissue free fatty acids isolated by aminopropyl bonded-phase columns. J Chromatogr 1988;428:221–228.
Das DK, Engelman RM, Rousou JA, et al. Pathophysiology of superoxide radical as potential mediator of reperfusion injury in pig heart. Basic Res Cardiol 1986;81:155–166.
Cordis GA, Engelman RM, Das DK. A novel computerassisted dual-wavelength monitoring approach for the improved rapid separation and estimation of adenine nucleotides and creatine phosphate by high performance liquid chromatography. J Chromatogr 1988;459:229–236.
Misra HP, Squatrito PM. The role of superoxide anion in peroxidase-catalyzed chemiluminescence of luminol. Arch Biochem Biophys 1982;215:59–65.
Liu X, Engelman RM, Agrawal HR, et al. Preservation of membrane phospholipids by propranolol, pindolol, and metoprolol: A novel mechanism of action of beta-blockers. J Mol Cell Cardiol 1991;23:1091–1100.
Wers SW, Shea MJ, Mitosos SE, et al. Reduction in the size of infarction by allopurinol in the ischemic-reperfused canine heart. Circulation 1986;73:518–524.
Otani H, Engelman RM, Rousou JA, et al. Cardiac performance during reperfusion improved by pretreatment with oxygen free radical scavengers. J Thorac Cardiovasc Surg 1986;91:290–295.
Zweier JL. Measurement of superoxide derived free radicals in the reperfused heart. J Biol Chem 1988;263:1353–1357.
Liu X, Prasad R, Engelman RM, et al. Role of ion on membrane phospholipid degradation in ischemic and reperfused rat heart. Am J Physiol 1990;259:H1101-H1107.
Myers ML, Bolli R, LeKich RF, et al. N-2 mercaptopropionylglycine improves recovery of myocardial function after reversible ischemia. J Am Coll Cardiol 1986;8:1161–1168.
Forman MB, Puett DW, Cates CU, et al. Glutathione redox pathway and reperfusion injury: Effect on N-acetylcysteine on infarct size and ventricular function. Circulation 1988; 78:202–213.
de Graeff PA, van Gilst WH, Bel K, et al. Concentrationdependent protection by captopril against myocardial damage during ischemia and reperfusion in a closed-chest pig model. J Cardiovasc Pharmacol 1987;9 (Suppl 2):S37-S42.
Brown EJ, Swinford RD, Shatkin BJ, et al. Massive reperfusion injury: Paradoxical effects of combined angiotensinconverting enzyme inhibition plus myocardial reperfusion (abstract). J Am Coll Cardiol1988;11:50A.
Chien KR, Han A, Sen A. Accumulation of unsaturated arachidonic acid in ischemic canine myocardium. Relationship to a phosphatidylcholine deacylation-reacylation cycle and the depletion of membrane phospholipids. Circ Res 1984; 54:313–322.
Das DK, Engelman RM, Rousou JA, et al. Role of membrane phospholipids in the myocardial injury introduced by ischemia and reperfusion. Am J Physiol 1986;251:H71-H79.
van Gilst WH, Scholtens E, de Graeff PA, et al. Differential influences of angiotensin converting-enzyme inhibitors on the coronary circulation. Circulation 1988;77 (suppl I): I24-I29.
Clough DP, Collis MG, Conway J, et al. Interaction of angiotensin converting enzyme inhibitors with the function of the sympathetic nervous system. Am J Cardiol 1982;49:1410.
Cushman DW, Wang FL, Fung WC, De Forrest JM. Differentiation of angiotensin-converting enzyme (ACE) inhibitors by their selective inhibition of ACE in physiologically important target organs. Am J Hypertens 1989;2:294–306.
Rademaker M, Shaw TRD, Williams BC, et al. Intravenous captopril treatment in patients with severe cardiac failure. Br Heart J 1986;55:187–190.
Li K, Chen XF. Protective effects of captopril and enalapril on myocardial ischemia and reperfusion damage of rat. J Mol Cell Cardiol 1987;19:909–915.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liu, X., Engelman, R.M., Rousou, J.A. et al. Attenuation of myocardial reperfusion injury by sulfhydryl-containing angiotensin converting enzyme inhibitors. Cardiovasc Drug Ther 6, 437–443 (1992). https://doi.org/10.1007/BF00054194
Issue Date:
DOI: https://doi.org/10.1007/BF00054194