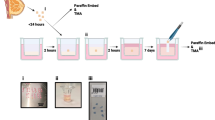
Transition from an epithelioid (e-) to a fibroblastic (f-) morphotype marks invasiveness in clinical and experimental cancer. To understand better the factors influencing such transitions, we have subcloned and manipulated mouse mammary gland (NMuMG) cell cultures and compared the invasive phenotype of multiple subclones in vitro and in vivo. Cell lines with an e-morphotype expressed E-cadherin homogeneously and were not invasive in vitro. Cells with an f-morphotype were E-cadherin-negative and became fully invasive in vitro upon expression of the ras oncogene. Invasive tumors were produced in nude mice after subcutaneous injection of e-type or f-type cells. These tumors showed cystic, glandular and undifferentiated structures. Tumors from f-type cells were E-cadherin-negative whereas e-type tumors stained heterogeneously in immunohistochemical preparations. Our observations demonstrate the impact of the micro-ecosystem on the invasive phenotype, with in vivo downregulation of E-cadherin and stimulation of the e- to f-morphotype transition.
Similar content being viewed by others
References
Jouanneau J, Tucker GC, Boyer B, Vallés AM and Thiery JP, 1991, Epithelial cell plasticity in neoplasia. Cancer Cells, 3, 525–9.
Van RoyF and Mareel M, 1992, Tumour invasion: effects of cell adhesion and motility. Trends Cell Biol, 2, 163–9.
Mareel M, Bracke M, Van Roy F and Vakaet L, 1993, Expression of E-cadherin in embryogenetic ingression and cancer invasion. Int J Dev Biol, 37, 227–35.
Stoler AB, Stenback F and Balmain A, 1993, The conversion of mouse skin squamous cell carcinomas to spindle cell carcinomas is a recessive event. J Cell Biol, 122, 1103–17.
Borén T, Falk P, Roth KA, Larson G and Normark S, 1993, Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science, 262, 1892–5.
Berg JW and Hutter RVP, 1995, Breast cancer. Cancer, 75 (Suppl), 257–69.
Sanford KK, Dunn TB, Westfall BB, Covalesky AB, Dupree LT and Earle WR, 1961, Sarcomatous change and maintenance of differentiation in long-term cultures of mouse mammary carcinoma. J Natl Cancer Inst, 26, 1139–83.
Dulbecco R, Henahan M, Bowman M, et al, 1981, Generation of fibroblast-like cells from cloned epithelial mammary cells in vitro: a possible new cell type. Proc Natl Acad Sci USA, 78, 2345–9.
Warburton MJ, Ferns SA, Hughes CM and Rudland PS, 1985, Characterization of rat mammary cell types in primary culture: lectins and antisera to basement membrane and intermediate filament proteins as indicators of cellular heterogeneity. J Cell Sci, 79, 287–304.
Sonnenberg A, Daams H, Calafat J and Hilgers J, 1986, In vitro differentiation and progression of mouse mammary tumor cells. Cancer Res, 46, 5913–22.
Vleminckx K, Vakaet L Jr, Mareel M, Fiers W and Van Roy F, 1991, Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell, 66, 107–19.
Nakanishi H, Taylor RM, Chrest FJ, et al. 1995, Progression of hormone-dependent adenocarcinoma cells to hormone-independent spindle carcinoma cells in vitro in a clonal spontaneous rat mammary tumor cell line. Cancer Res, 55, 399–407.
Reichmann E, Schwarz H, Deiner EM, et al. 1992, Activation of an inducible c-fosER fusion protein causes loss of epithelial polarity and triggers epithelialfibroblastoid cell conversion. Cell, 71, 1103–16.
Mareel MM, De Bruyne GK, Sonnenberg A and Hilgers J, 1986, Experimental results on invasiveness of mouse mammary cells: clinical implications? In: Hollmann KH and Verley JM, eds. New Frontiers in Mammary Pathology. Martinus Nijhoff Publishers, Boston, pp. 351–62.
Owens RB, Smith HS and Hackett AJ, 1974, Epithelial cell cultures from normal glandular tissue of mice. J Natl Cancer Inst, 53, 261–9.
Schaeffer WI, 1983, Usage of vertebrate, invertebrate and plant cell, tissue and organ culture terminology. TCA Report, 17, 19–23.
Vandenbossche GMR, De Bruyne GK, Bruyneel EA, et al. 1994, Microencapsulation of MDCK-ras-e cells prevents loss of the E-cadherin invasion suppressor function in vivo. Int J Cancer, 57, 73–80.
Mareel MM, Van Roy FM, Messiaen LM, Boghaert ER and Bruyneel EA, 1987, Qualitative and quantitative analysis of tumour invasion in vivo and in vitro. J Cell Sci Suppl, 8, 141–63.
Mareel MM, Van Roy FM and Bracke ME, 1993, How and when do tumor cells metastasize? Crit Rev Oncogenesis, 4, 559–94.
Mareel MM, Behrens J, Birchmeier W, et al. 1991, Downregulation of E-cadherin expression in Madin Darby canine kidney (MDCK) cells inside nude mice tumors. Int J Cancer, 47, 922–8.
Van Roy FM, Messiaen L, Liebaut G, et al. 1986, Invasiveness and metastatic capability of rat fibroblast-like cells before and after transfection with immortalizing and transforming genes. Cancer Res, 46, 4787–95.
Van Muyen GNP, Ruiter DJ, Franke WW, et al. 1986, Cell type heterogeneity of cytokeratin expression in complex epithelia and carcinomas as demonstrated by monoclonal antibodies specific for cytokeratins nos. 4 and 13. Exp Cell Res, 162, 97–113.
Mareel M, Kint J and Meyvisch C, 1979, Methods of study of the invasion of malignant C3H mouse fibroblasts into embryonic chick heart in vitro. Virchows Arch B Cell Pathol, 30, 95–111.
Mareel MM, De Bruyne GK, Vandesande F and Dragonetti C, 1981, Immunohistochemical study of embryonic chick heart invaded by malignant cells in three-dimensional culture. Inv Metastasis, 1, 195–204.
Bracke ME, Van Cauwenberge RM-L and Mareel MM, 1984, (+)-Catechin inhibits the invasion of malignant fibrosarcoma cells into chick heart in vitro. Clin Exp Metastasis, 2, 161–70.
Mareel M, Coopman P, Dragonetti C, et al. 1988, Spontaneous and oncogene-mediated acquisition of the invasive phenotype by cells in culture. In: Nicolson G and Fidler I, eds. UCLA Symposium on Molecular and Cellular Biology, Tumor Progression and Metastasis, 78, 179–88.
Frith CH and Ward JM, 1988, Color Atlas of Neoplastic and Non-Noplastic Lesions in Aging Mice. Elsevier Science Publishers BV, Amsterdam.
Mareel M, Bracke M, Bruyneel E, Van Larebeke N, Bourgois L and De Mets M, 1990, Assays in vitro and in vivo for invasion and metastasis: application to drug testing. In: Etiévant C, Cros J, Rustum YM, eds. New Concepts in Cancer Metastasis, Oncogenes and Growth Factors, P Fabre Monograph Series, Vol 3. McMillan Press Ltd, pp. 148-65.
David G and Bernfield M, 1982, Defective basal lamina formation by transformed mammary epithelial cells: a reduced effect of collagen on basal lamina (heparan sulfate-rich) proteoglycan degradation. J Cell Physiol, 110, 56–62.
David G and Van den Berghe H, 1985, Heparan sulfate-chondroitin sulfate hybrid proteoglycan of the cell surface and basement membrane of mouse mammary epithelial cells. J Biol Chem, 260, 11067–74.
Dulbecco R, Armstrong B and Allen R, 1988, Reversion toward an earlier stage of differentiation and loss of polarity during progression of n-methyl-n-nitrosourea-induced rat mammary tumors. Proc Natl Acad Sci USA, 85, 9292–6.
Gabbert HE, Meier S, Gerharz CD and Hommel G, 1992, Tumor-cell dissociation at the invasion front: a new prognostic parameter in gastric cancer patients. Int J Cancer, 50, 202–7.
Mareel M, Van Roy F and De Baetselier P, 1990, The invasive phenotypes. Cancer Metastasis Rev, 9, 45–62.
Mareel MM, De Baetselier P and Van Roy FM, 1991, Mechanisms of Invasion and Metastasis. CRC Press, Boca Raton, Ann Arbor, Boston. ISBN 0–8493–6254–7.
David G, Van der Schueren B and Bernfield M, 1981, Basal lamina formation by normal and transformed mouse mammary epithelial cells duplicated in vitro. J Natl Cancer Inst, 67, 719–28.
Mareel M, Bracke M and Van Roy F, 1994, Invasion promoter versus invasion suppressor molecules: the paradigm of E-cadherin. Mol Biol Rep, 19, 45–67.
Behrens J, Frixen U, Schipper J, Weidner M and Birchmeier W, 1992, Cell adhesion in invasion and metastasis. Semin Cell Biol, 3, 169–78.
Takeichi M, 1991, Cadherin cell adhesion receptors as a morphogenetic regulator. Science, 251, 1451–5.
Vleminckx KL, Deman JJ, Bruyneel EA, et al. 1994, Enlarged cell-associated proteoglycans abolish Ecadherin functionality in invasive tumor cells. Cancer Res, 54, 873–7.
Oka H, Shiozaki H, Kobayashi K, et al. 1993, Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res, 53, 1696–701.
Becker KF, Atkinson MJ, Reich U, et al. 1993, Exon skipping in the E-cadherin gene transcript in metastatic human gastric carcinomas. Hum Mol Genet, 2, 803–4.
Geiger B, Volberg T, Ginsberg D, Bitzur S and Sabanay I, 1990, Broad spectrum pan-cadherin antibodies, reactive with the c-terminal 24 amino acid residues of n-cadherin. J Cell Sci, 97, 607–14.
Hynes NE, Jaggi R, Kozma SC, et al. 1985, New acceptor cell for transfected genomic DNA: oncogene transfer into a mouse mammary epithelial cell line. Mol Cell Biol, 5, 268–72.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Van den Broecke, C., Vleminckx, K., De Bruyne, G. et al. Morphotypic plasticity in vitro and in nude mice of epithelial mouse mammary cells (NMuMG) displaying an epithelioid (e) or a fibroblastic (f) morphotype in culture. Clin Exp Metast 14, 282–296 (1996). https://doi.org/10.1007/BF00053902
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00053902