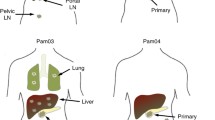
Understanding the genetic elements controlling the process of tumor metastasis to distant organ sites such as the liver may be the key to improving survivorship from colon cancer. By using standard cytogenetic techniques in combination with comparative genomic hybridization, multiple genetic imbalances within three human colon cancer cell lines previously selected for differences in liver-metastatic behavior were identified. The entire genome of one poorly metastatic cell line (KM12C) was compared directly with that of two highly metastatic cell lines (KM12SM, KM12L4A) derived from it. A number of chromosomal gains (8q, 12g15, 20q11.2) and losses (5p13, 6p21.3, 18) were common to all three cell lines and are likely related to early tumor development rather than to the selection process used to generate cell lines of increased metastatic potential. Chromosomal imbalances detected only in the highly metastatic cell lines were also observed. KM12SM showed losses of portions of 2p22, 2824.3→2q32.2, 4p15.3→cen, 4q24 without the 13q and 15q22.3 gains noted for KM12C. Both gains (1p31.3→1p21, 2822→2q33, 3cen→3q26.2, 5q14→5q23, 6cen→6q23) and losses (16p, 17p, 17q, 19p, 19q, 22q) were observed for KM12L4A but not for the other two cell lines. Identification of these alterations provides valuable insight into the process of experimental liver metastasis and is a first step towards mapping genes linked to the terminal phases of human colon cancer progression.
Similar content being viewed by others
References
Boring CC, Squires T, Tong T and Montgomery S, 1994, Cancer statistics. CA Cancer J Clin, 44, 7–26.
Goyette MC, Cho K, Fasching CL et al, 1992, Progression of colorectal cancer is associated with multiple tumor suppressor gene defects but inhibition of tumorigenicity is accomplished by correction of any single defect via chromosome transfer. Molec Cell Biol, 12, 1387–95.
Yeatman TJ and Nicolson GL, 1993, Molecular basis of tumor progression: mechanisms of organ-specific tumor metastasis. Semin Surg Oncol, 9, 256–63.
Liotta LA, 1992, Cancer cell invasion and metastasis. Sci Am, 266, 54–63.
Nicolson GL, 1989, Metastatic tumor cell interactions with endothelium, basement membrane, and tissue. Curr Opin Cell Biol, 1, 1009–19.
Nicolson GL, Inoue T, Van Pelt C and Cavanaugh PG, 1990, Differential expression of a Mr 90,000 cell surface transferrin-related glycoprotein on murine B16 metastatic melanoma sublines selected for enhanced brain or ovary colonization. Cancer Res, 50, 515–20.
Morikawa K, Walker S, Jessup JM and Fidler IJ, 1988, In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res, 48, 1943–8.
Morikawa K, Walker S, Nakajima M, Pathak S, Jessupp JM and Fidler IJ, 1988, Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res, 48, 6863–71.
Radinsky R, Risin S, Fan D et al, 1995, Level and function of epidermal growth factor receptor predict the metastatic potential of human colon carcinoma cells. Clin Cancer Res, 1, 19–31.
Kallioniemi A, Kallioniemi O, Sudar D et al, 1992, Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science, 258, 5083.
Tanner MM, Tirkkonen M, Kallioniemi A et al, 1994, Increased copy number at 20g13 in breast cancer: defining the critical region and exclusion of candidate genes. Cancer Res, 54, 4257–60.
Knapek DF, Serrano M, Beach D, Trono D and Walker CL, 1995, Association of rat p15INK4B/p15INK4 deletions with monosomy 5 in kidney epithelial cell lines but not primary renal tumors. Cancer Res, 55, 1607–12.
Cher ML, MacGrogan D, Bookstein R, Brown JA, Jenkins RB and Jensen RH, 1994, Comparative genomic hybridization, allelic imbalance, and fluorescence in situ hybridization on chromosome 8 in prostate cancer. Genes Chromosom Cancer, 11, 153–62.
Bardi G, Johansson B, Pandis N et al, 1993, Cytogenetic aberrations in colorectal adenocarcinomas and their correlation with clinicopathologic features. Cancer 71, 306–14.
Kern SE, Fearon ER and Tersmette KWF, 1989, Allelic loss in colorectal carcinoma. JAMA, 261, 3099–103.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeatman, T.J., Cher, M.L., Mao, W. et al. Identification of genetic alterations associated with the process of human experimental colon cancer liver metastasis in the nude mouse. Clin Exp Metast 14, 246–252 (1996). https://doi.org/10.1007/BF00053898
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00053898