Abstract
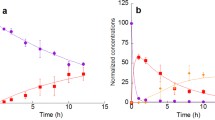
Elevated concentrations of S(IV) and formaldehyde were observed in fog- and cloudwater at sites in California. The highest concentrations (up to 3 mM S(IV) and 0.7 mM CH2O) were measured at Bakersfield, during a prolonged period of repeated fog. In Bakersfield [S(IV)] generally exceeded [CH2O], while in the Los Angeles area the reverse was observed. The lowest concentrations of both species were observed at marine and high altitude sites away from local emissions. Equilibrium computations indicate that high concentrations of S(IV) cannot be achieved without the formation of S(IV)-RCHO adducts.
Similar content being viewed by others
References
Bell, R. P., 1966, The reversible hydration of carbonyl compounds, in V.Gold (ed.),Advances in Physical Organic Chemistry, vol. 4, Academic Press, London, pp. 1–29.
Bell, R. P., Evans, F. R. S., and Evans, P. G., 1966, Kinetics of the dehydration of methylene glycol in aqueous solution,Proc. Roy. Soc. A. 291, 297–323.
Bordwell, F. G., 1963,Organic Chemistry, Macmillan, New York.
Boyce, S. D. and Hoffmann, M. R., 1984. Kinetics and mechanism of the formation of hydroxy-methanesulfonic acid at low pH,J. Phys. Chem., in press.
Buschmann, H. J., Fulderner, H. H., and Knoche, W., 1980, The reversible hydration of carbonyl compounds in aqueous solution. Part I, the ketol gem-diol equilibrium,Ber. Bunsenges Phys. Chem. 84, 41–44.
California Air Resources Board (CARB), 1982,Emissions Inventory, California Air Resources Board, Sacramento, California.
Calvert, J. G. and Pitts, J. N. Jr., 1966,Photochemistry, John Wiley, New York.
Calvert, J. G., 1980, The homogeneous chemistry of formaldehyde generation and destruction within the atmosphere, inProceedings of the NATO Advanced Study Institute on Atmospheric Ozone: Its Variation and Human Influences, Federal Aviation Administration, Washington, D.C., pp. 153–190.
Chameides, W. L. and Davis, D. D., 1983. Aqueous-phase source of formic acid in clouds,Nature 304, 427–429.
Cleveland, W. S., Graedel, T. E., and Kleiner, B., 1977, Urban formaldehyde: Observed correlation with source emissions and photochemistry,Atmos. Environ. 11, 357–360.
Dasgupta, P. K., DeCesare, K., and Ullrey, J. C., 1980, Determination of atmospheric sulfur dioxide without tetrachlormercurate(II) and the mechanism of the Schiff Reactions,Anal. Chem. 52, 1912–1922.
Demerjian, K. L., Kerr, J. A., and Calvert, J. G., 1974, The mechanism of photochemical smog formation,Adv. Environ. Sci. Technol. 4, 1–262.
Eatough, D. J. and Hansen, L. D., 1983, Organic and inorganic S(IV) compounds in airborne particulate matter,Adv. Environ. Sci. Technol. 12, 221–268.
Fortune, C. R. and Dellinger, B., 1982, Stabilization and analysis of S(IV) aerosols in environmental samples,Environ. Sci. Tech. 16, 62–66.
Grosjean, D., 1982, Formaldehyde and other carbonyls in Los Angeles ambient air,Environ. Sci. Technol. 16, 254–262.
Grosjean, D. and Wright, B., 1983, Carbonyls in urban fog, ice fog, cloudwater and rainwater,Atmos. Environ. 17, 2093–2096.
Hering, S. V. and Blumenthal, D. L., 1983, Field comparison of fog/cloud water collectors: Preliminary results,Proc. APCA Specialty Conference on the Meteorology of Acid Deposition, Hartford, Connecticut, October, 1983.
Hoffmann, M. R. and Boyce, S. D., 1983, Catalytic autoxidation of aqueous sulfur dioxide in relationship to atmospheric systems,Adv. Environ. Sci. Technol. 12, 149–189.
Humphrey, R. E., Ward, M. H., and Hinze, W., 1970, Spectrophotometric determination of sulfite with 4,4-Dithiopyridine and 5,5′-Dithiobis (2-Nitrobenzoic acid),Anal. Chem. 42, 698–702.
Izatt, R. M., Eatough, D. J., Lee, M. L., Major, T., Richter, B. E., Hansen, L. D., Meisenheimer, R. G., and Fischer, J. W., 1978, The formation of inorganic and organic S(IV) species in aerosols,Proc. 4th Joint Conf. on Sensing Environ. Pollutants, Amer. Chem. Soc., Washington, D.C., pp. 821–824.
Jacob, D. J. and Hoffmann, M. R., 1983, A dynamic model for the production of H+, NO3 −, and SO4 2− in urban fog,J. Geophys. Res. 88C, 6611–6621.
Jacob, D. J., Wang, R-F. T., and Flagan, R. C., 1984, Fogwater collector design and characterization,Environ. Sci. Technol., in press.
Jacob, D. J., Waldman, J. M., Munger, J. W., and Hoffmann, M. R., 1984, A field investigation of physical and chemical mechanisms affecting pollutant concentrations in fog droplets,Tellus, in press.
Klippel, W. and Warneck, P., 1978, Formaldehyde in rain water and on the atmospheric aerosol,Geophys. Res. Lett. 5, 177–179.
Klippel, W. and Warneck, 1980, The formaldehyde content of the atmospheric aerosol,Atmos. Environ. 14, 809–818.
Ledbury, W. and Blair, E. W., 1925, The partial formaldehyde vapour pressure of aqueous solutions of formaldehyde. Part II.J. Am. Chem. Soc. 127, 2832–2839.
McArdle, J. V. and Hoffmann, M. R., 1983, Kinetics and mechanism of the oxidation of aquated sulfur dioxide by hydrogen peroxide at low pH,J. Phys. Chem. 87, 5425–5429.
Munger, J. W., Jacob, D. J., Waldman, J. M., and Hoffmann, M. R., 1983, Fogwater chemistry in an urban atmosphere,J. Geophys. Res. 88, 5109–5121.
Nash, T., 1953, The colorimetric estimation of formaldehyde by means of the Hantzsch Reaction,Biochem. J. 55, 416–421.
National Research Council (NRC), 1981,Formaldehyde and Other Aldehydes, National Academy Press, Washington, D.C.
Niki, H., Maker, P. D., Savage, C. M., and Breitenbach, L. P., 1978, Mechanism for hydroxyl radical initiated oxidation of olefin-nitric oxide mixtures in parts per million concentrations,J. Phys. Chem. 82, 135–137.
Reible, D. D., Shair, F. H., Smith, T. B., Lehrman, D. E., 1983, The origin and fates of air pollutants in California's San Joaquin Valley I. Winter.Atmos. Environ., in press.
Reitz, E. B., 1980, The stabilization of small concentrations of formaldehyde in aqueous solutions,Anal. Lett. 13, 1073–1084.
Richards, L. W., Anderson, J. A., Blumenthal, D. L., McDonald, J. A., Kok, G. L., and Lazrus, A. L., 1983, Hydrogen peroxide and sulfur(IV) in Los Angeles cloudwater,Atmos. Environ. 17, 911–914.
Roberts, J. D., Stewart, R., and Caserio, M. C., 1971,Organic Chemistry, W.A. Benjamin, Menlo Park, CA.
Sillén, G. L. and Martell, A. E., 1964,Stability Constants of Metal-ion Complexes, special publication No. 17, Chemical Society, London.
Smith, R. H., 1978, Rate constant and activation energy for the gaseous reaction between hydroxyl and formaldehyde,Int. J. Chem. Kinet. 10, 519–528.
Smith, R. V. and Erhardt, P. W., 1975, Nash determination for formaldehyde in the presence of bisulfite,Anal. Chem. 47, 2462–2454.
Sørensen, P. E. and Andersen, V. S., 1970, The formaldehyde-hydrogen sulphite system in alkaline aqueous solution. Kinetics, mechanisms, and equilibria,Acta Chem. Scand. 24, 1301–1306.
Su, F., Calvert, J. G., and Shaw, J. H., 1979, Mechanism of the photooxidation of gaseous formaldehyde,J. Phys. Chem. 83, 3185–3191.
Stewart, T. D. and Donnally, L. H., 1932, The aldehyde bisulfite compounds II. The effect of varying hydrogen ion and of varying temperature upon the equilibrium between benzaldehyde and bisulfite ion.J. Am. Chem. Soc. 54, 3555–3558.
Waldman, J. M., Munger, J. W., Jacob, D. J., Flagan, R. C., Morgan, J. J., and Hoffmann, M. R., 1982, Chemical composition of acid fog.,Science 218, 677–680.
Zafiriou, O. C., Alford, J., Herrera, M., Peltzer, E. T., and Gagosian, R. B., 1980, Formaldehyde in remote marine air and rain: Flux measurements and estimates,Geophys. Res. Lett. 5, 341–344.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Munger, J.W., Jacob, D.J. & Hoffmann, M.R. The occurrence of bisulfite-aldehyde addition products in fog- and cloudwater. J Atmos Chem 1, 335–350 (1984). https://doi.org/10.1007/BF00053799
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00053799