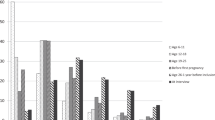
The association with breast cancer of menstrual and reproductive events, family history of breast cancer, and body size have been studied on two cohorts of 6,706 volunteers on the island of Guernsey (United Kingdom), 168 of whom had breast cancer detected during follow-up. The median follow-up time of the non-cases was 21 years in the first study and 10 years in the second. A time-dependent Cox regression model was fitted to the data with age as the time-dependent variable in order to represent the effect of changing menopausal status. Other variables examined in the model were age at menarche, parity, age at first birth, family history of breast cancer, height, weight (both directly measured), relative weight (weight [kg]/height[m]), and Quetelet's body mass index (weight[kg]/height[m]2). Interactions between age and all other covariates also were examined. Family history was found to be the most important risk factor for women aged less than 51 years (relative risk [RR]=3.5, 95 percent confidence interval [CI]=2.0–6.0), and intervals between menarche and first birth longer than 14 years were found to increase significantly the risk of breast cancer in women older than 61 years (RR=2.4, CI=1.3–4.4). Height was the only indicator of body size which was associated significantly with risk of breast cancer, the estimated regression coefficient indicating an increase in risk of about 70 percent for women on the 90th centile of height relative to those on the 10th centile. A survey of the literature showed that the association between risk of breast cancer and height was found in those studies which used direct measurements of height but not in others which used self-reported values.
Similar content being viewed by others
References
Vatten LJ, Kvinnsland S. Body height and risk of breast cancer. A prospective study of 23,831 Norwegian women. Br J Cancer 1990; 61: 881–5.
Swanson CA, Jones DY, Schatzkin A, Brinton L, Ziegler R. Breast cancer risk assessed by anthropometry in the NHANES I epidemiological follow-up study. Cancer Res 1988; 48: 5363–7.
Albanes D, Jones DY, Schatzkin A, Micozzi MS, Taylor PR. Adult stature and risk cancer. Cancer Res 1988; 48: 1658–62.
Swanson CA, Brinton LA, Taylor PR, Licitra LM, Ziegler RG, Schairer C. Body size and breast cancer risk assessed in women participating in the Breast Cancer Detection Demonstration Project. Am J Epidemiol 1989; 130: 1133–41.
Tretli S. Height and weight in relation to breast cancer morbidity and mortality. A prospective study of 570,000 women in Norway. Int J Cancer 1989; 44: 23–30.
Hsieh C-c, Trichopoulos D, Katsouyanni K, Yuasa S. Age at menarche, age at menopause, height and obesity as risk factors for breast cancer: associations and interactions in an international case-control study. Int J Cancer 1990; 46: 796–800.
Bruning PF, Bonfrer JMG, Hart AAM, et al. Body measurements, estrogen availability and the risk of human breast cancer: a case-control study. Int J Cancer 1992; 51: 14–9.
Vatten LJ, Kvikstad A, Nymoen EH. Incidence and mortality of breast cancer related to body height and living conditions during childhood and adolescence. Enr J Cancer 1992; 28: 128–31.
Bulbrook RD, Hayward JL, Spicer CC. Relation between urinary androgen and corticoid excretion and subsequent breast cancer. Lancet 1971; 2: 395–8.
Wang DY, De Stavola BL, Bulbrook RD, et al. The permanent effect of reproductive events on blood prolactin levels and its relation to breast cancer risk: a population study of postmenopausal women. Eur J Clin Oncol 1988; 24: 1225–31.
Feinleib M. Breast cancer and artificial menopause: a cohort study. JNCI 1968; 41: 315–21.
Cox DR, Oakes D. Analysis of Survival Data. London: Chapman and Hall, 1984.
Preston DL, Lubin JH, Pierce DA. EPICURE User's Guide. Seattle, WA: HiroSoft International, 1991.
Computing Resource Center. STATA Reference Manual. Santa Monica, CA: CRC, 1992.
Albanes D, Brown C. Relative weight, height, and risk of breast cancer (Letter). JAMA 1990; 263: 3148.
Fleming TR, Harrington DP. Counting Processes and Survival Analysis. New York: John Wiley, 1991.
Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: parsimonious parametric modelling. JR Stat Soc, submitted.
De Waard A, Trichopoulos D. A unifying concept of the aetiology of breast cancer. Int J Cancer 1988; 41: 666–9.
Kampert JB, Whittemore AS, Paffenbarger RS. Combined effect of childbearing, menstrual events, and body size on age-specific breast cancer risk. Am J Epidemiol 1988; 128: 962–79.
Pathak DR, Whittemore AS. Combined effects of body size, parity and menstrual events on breasts cancer incidence in seven countries. Am J Epidemiol 1992; 135: 153–68.
Micozzi MS. Nutrition, body size and breast cancer. Yearbook Phys Anthropol 1983; 28: 175–206.
Moisan J, Meyer F, Gingras S. A nested case-control study of the correlates of early menarche. Am J Epidemiol 1990; 132: 953–61.
Key TJA, Pike MC. The role of oestrogens and progestagens in the epidemiology and prevention of breast cancer. Eur J Cancer 1988; 28: 29–43.
Le Marchand L, Kolonel LN, Earle ME, Mi M-P. Body size at different periods of life and breast cancer risk. Am J Epidemiol 1988; 128: 137–52.
London S, Colditz GA, Stampfer MJ, Willett WC, Rosner B, Speizer FE. Prospective study of relative weight, height, and risk of breast cancer. JAMA 1989; 262: 2853–8.
Ballard-Barbash R, Schatzkin A, Taylor P, Kahle LL. Association of change in body mass with breast cancer. Cancer Res 1990; 50: 2152–5.
Folsom AR, Kaye SA, Prineas RJ, Potter JD, Gapstur SM, Wallace RB. Increased incidence of carcinoma of the breast associated with abdominal adiposity in postmenopausal women. Am J Epidemiol 1990; 131: 794–803.
Sullivan Pepe M, Self SG, Prentice RL. Further results on covariate measurement errors in cohort studies with time to response data. Stat Med 1989; 8: 1167–78.
Armstrong BG. The effects of measurements errors on relative risk regression. Am J Epidemiol 1990; 312: 1176–84.
Stewart AW, Jackson RT, Ford MA, Beaglehole R. Underestimation of relative weight by use of selfreported height and weight. Am J Epidemiol 1987; 125: 122–6.
Le Marchand L, Yoshizawa CN, Nomura AMY. Validation of body size information on driver's licenses. Am J Epidemiol 1988; 128: 874–7.
Waaler HT, Lund E. Association between body height and death from breast cancer. Br J Cancer 1983; 48: 149–50.
Ewertz M. Influence of non-contraceptive exogenous and endogenous sex hormones on breast cancer risk in Denmark. Int J Cancer 1988; 42: 832–8.
Parazzini F, La Vecchia C, Negri E, Bruzzi P, Palli D, Brule P. Anthropometric variables and risk of breast cancer. Int J Cancer 1990; 45: 397–402.
Lund E, Adami H-O, Bergstrom R, Meirik O. Anthropometric measures and breast cancer in young women. Cancer Causes Control 1990; 1: 169–72.
La Vechia C, Negri E, Parazzini F, et al. Height and cancer risk in a network of case-control studies from northern Italy. Int J Cancer 1990; 45: 275–9.
Marr LJ. A History of the Bailiwick of Guernsey. London and Chichester: Phillimore and Co, 1982.
Mettlin C, Croghan I, Natarajan N, Lane W. The association of age and familial risk in a case-control study of breast cancer. Am J Epidemiol 1990; 131: 973–83.
Claus EB, Risch NJ, Thompson WD. Age at onset as an indicator of familial risk of breast cancer. Am J Epidemiol 1990; 131: 961–72.
Moolgavkar SH, Day NE, Stevens RG. Two-stage model for carcinogenesis: epidemiology of breast cancer in females. JNCI 1980; 65: 559–69.
Davey Smith G, Phillips AN. Confounding in epidemiological studies: Why “independent” effects may not be all they seem. Br Med J 1992; 305: 757–9.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
De Stavola, B.L., Wang, D.Y., Allen, D.S. et al. The association of height, weight, menstrual and reproductive events with breast cancer: results from two prospective studies on the island of Guernsey (United Kingdom). Cancer Causes Control 4, 331–340 (1993). https://doi.org/10.1007/BF00051335
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00051335