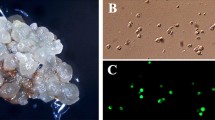
Abstract
Protoplasts were isolated from aseptic shoot cultures of commercial cultivars ofBrassica napus, B. oleracea andB. campestris, and from the six ‘rapid-cycling brassica species’. Of the rapid-cycling species, onlyB. napus responded well to the culture conditions used; 2% of protoplasts formed calli and up to 5% of calli regenerated shoots. Regeneration was also achieved from commercial cultivars ofB. napus andB. oleracea. For these two species the plating density, time of dilution with fresh medium and the composition of the shoot-inducing medium were all found to have an important influence on the efficiency of plant regeneration. Both responded better to maltose than to sucrose-based media. Under the optimum conditionsB. napus showed a plating efficiency of 7.8% and shooting efficiency of 17%; forB. oleracea the figures were 2% and 56%, respectively.
Similar content being viewed by others
Abbreviations
- BAP:
-
6-benzylaminopurine
- NAA:
-
α-naphthaleneacetic acid
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
References
Williams PH, Hill CB (1986) Rapid-cycling populations ofBrassica. Science 232: 1385–1389
MacDonald MV, Ingram DS (1984) The use of tissue culture in oilseed rape breeding. Aspects Appl Biol 6: 37–48
Charest PJ, Holbrook LA, Gabard J, Iyer VN, Miki BL (1988)Agrobacterium-mediated transformation ofBrassica napus L. Theor Appl Genet 75: 438–445
Bidney DL, Shepard JF, Kaleikau E (1983) Regeneration of plants from mesophyll protoplasts ofBrassica oleracea. Protoplasma 117: 89–92
Glimelius K (1984) High growth rate and regeneration capacity of hypocotyl protoplasts in some Brassicaceae. Physiol Plant 61: 38–44
Pelletier G, Primard C, Vedel F, Chetrit P, Remy R, Rouselle P, Renard M (1983) Intergeneric cytoplasmic hybridisation in Cruciferae by protoplast fusion. Mol Gen Genet 191: 244–250
Barsby TL, Yarrow SA, Shepard JF (1986) A rapid and efficient alternative procedure for the regeneration of plants from hypocotyl protoplasts ofBrassica napus. Plant Cell Rep 5: 101–103
Klimaszewska K, Keller WA (1987) Plant regeneration from stem cortex protoplasts ofBrassica napus. Plant Cell Tissue Organ Culture 8: 225–233
Xu ZH, Davey MR, Cocking EC (1982) Plant regeneration from root protoplasts ofBrassica. Plant Sci Lett 24: 117–121
Schenck HR, Robbelen G (1982) Somatic hybrids by fusion of protoplasts fromBrassica oleracea andB. campestris. Z Pflanzenzuchtg 89: 278–288
Terada R, Yamashita Y, Nishibayash S, Shimamoto K (1987) Somatic hybrids betweenBrassica oleracea andB. campestris: selection by the use of iodoacetate inactivation and regeneration ability. Theor Appl Genet 73: 379–384
Toriyama K, Hinata K, Kameya T (1987) Production of somatic hybrid plants ‘Brassicomoricandia’ through protoplast fusion betweenMoricandia arvensis andBrassica oleracea. Plant Sci 48: 123–128
Toriyama K, Kameya T, Hinata K (1987) Selection of a universal hybridiser inSinapis turgida Del. and regeneration of plantlets from somatic hybrids withBrassica species. Planta 170: 308–312
Gleba YY, Hoffmann F (1980) ‘Arabidobrassica’: A novel plant obtained by protoplast fusion. Planta 149: 112–117
Aslam F (1988) Haploid Production in Rapid-cyclingBrassica napus andB. campestris. PhD thesis, University of Cambridge, Cambridge
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497
Menczel L, Wolfe K (1984) High frequency of fusion induced in freely suspended protoplast mixtures by polyethylene glycol and dimethylsulfoxide at high pH. Plant Cell Rep 3: 196–198
Nitsche JP, Nitsche C (1969) Haploid plants from pollen grains. Science 163: 85–87
Hunter C (1988) Plant Regeneration from Microspores of Barley,Hordeum vulgare. PhD thesis, University of London, Wye College, Ashford, Kent
Caboche M (1980) Nutritional requirements of protoplast-derived haploid cells grown at low densities in liquid media. Planta 149: 7–18
Strickland SG, Nichol JW, McCall CM, Stuart DA (1987) Effect of carbohydrate source on alfalfa somatic embryogenesis. Plant Sci 48: 113–121
Kruger NJ, ap Rees T (1983) Maltose metabolism by pea chloroplasts. Plant 158: 179–184
Stitt M, Steup M (1985) Starch and sucrose degradation. In: Douce R, Day DA (Eds) Encyclopedia of Plant Physiology, Vol. 18. Springer-Verlag, Berlin
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Loudon, P.T., Nelson, R.S. & Ingram, D.S. Studies of protoplast culture and plant regeneration from commercial and rapid-cyclingBrassica species. Plant Cell Tiss Organ Cult 19, 213–224 (1989). https://doi.org/10.1007/BF00043348
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00043348