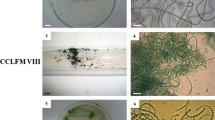
Abstract
A simple and rapid method for obtaining axenic plant material has been devised where antibiotic-containing paper disks are applied to nutrient agarose or agar plates upon which small pieces of plant have been spread. By applying multiple disks to the agarose plate, susceptibility to a large number of antibiotics can be tested simultaneously. Clear zones are produced around those disks where contaminating bacteria are susceptible. Plant pieces are then removed from the clear zones and separately tested for sterility to identify the axenic pieces. The method has been successfully applied to multicellular marine algae (e.g.Enteromorpha, Porphyra, laminaria, Gracilaria andAgardhiella). Pieces fromAgardhiella plants survive better on nutrient medium solidified with agarose when α-naphthaleneacetic acid and zeatin are present in the medium.
Similar content being viewed by others
References
Barry, AL, Thornsberry, C (1980) Susceptibility testing: diffusion test procedures. In: Lennette, EH (ed) Manual of Clinical Microbiology, 3rd. edit. Amer Soc Microbiol, Washington DC, pp463–473
Bauer, AW, Kirby, WMM, Sherris, JC, Turck, M (1966) Antibiotic susceptibility testing by a standardized single disk method. Amer J Clin Pathol 45: 493–496
Bradley PM, Pesano RL (1980) Algal sheath removal and axenic algae cultures. J Cell Biol 87: 228a
Capo, T (1981) Laboratory culture ofAgardhiella subulata (Rhodophyta), strain A1. Biol Bull 161: 338.
Fries, L (1963) On the cultivation of axenic red algae. Physiol Plant 16: 695–708
George, EF, Sherrington, PD (1984) Plant Propagation by Tissue Culture: handbook and directory of commercial laboratories. Exegetics Ltd., Hants UK, pp 91–93
Guillard, RRL, Ryther, JH (1962) Studies on marine planktonic diatoms I.Cyclotella nana Hustedt andDetonula confervacea (Cleve) Gran. Can J Microbiol 8: 229–239
Lewin, J (1966) Silicon metabolism in diatoms V. Germanium dioxide, a specific inhibitor of diatom growth. Phycologia 6: 1–12
Polne-Fuller, M, Gibor, A (1984) Developmental studies inPorphyra. I—Blade differentiation inPorphyra perforata as expressed by morphology, enzymatic digestion, and protoplast regeneration. J Phycol 20: 609–616
Provasoli, L, Pintner, IJ (1980) Bacteria induced polymorphism in an axenic laboratory strain ofUlva lactuca (Chlorophyceae). J Phycol 16: 196–201
Provasoli, L, McLaughlin, JJA, Droop, MR (1957) The development of artificial media for marine algae. Arch Microbiol 25: 392–428
Saga, N (1986) Pure culture of algae. [In Japanese]. In: Yamada, Y, Okada, Y (eds) Plant Biotechnology. Tokyo Kagaku Dojin, Tokyo, pp 55–71
Saga, N, Sakai, Y (1982) A new method for pure culture of macroscopic algae, the one step selection method. Jap J Phycol 30: 40–43
Schiewer, U (1969) Untersuchungen zur Sterilisation von mehrzelligen Brackwasseralgen mit Hilfe von Antibiotika, Sulfonamiden und Fungiziden. Int Rev ges Hydrobiol 54: 609–617
Zobell, CE (1941) Studies on marine bacteria I. The cultural requirements of heterotrophic aerobes. J Marine Res 4: 42–75
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bradley, P.M., Cheney, D.P. & Saga, N. One step antibiotic disk method for obtaining axenic cultures of multicellular marine algae. Plant Cell Tiss Organ Cult 12, 55–60 (1988). https://doi.org/10.1007/BF00043107
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00043107