Abstract
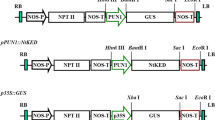
Osmotin is a small (24 kDa), basic, pathogenesis-related protein, that accumulates during adaptation of tobacco (Nicotiana tabacum) cells to osmotic stress. There are more than 10 inducers that activate the osmotin gene in various plant tissues. The osmotin promoter contains several sequences bearing a high degree of similarity to ABRE, as-1 and E-8 cis element sequences. Gel retardation studies indicated the presence of at least two regions in the osmotin promoter that show specific interactions with nuclear factors isolated from cultured cells or leaves. The abundance of these binding factors increased in response to salt, ABA and ethylene. Nuclear factors protected a 35 bp sequence of the promoter from DNase I digestion. Different 5′ deletions of the osmotin promoter cloned into a promoter-less GUS-NOS plasmid (pBI 201) were used in transient expression studies with a Biolistic gun. The transient expression studies revealed the presence of three distinct regions in the osmotin promoter. The promoter sequence from −108 to −248 bp is absolutely required for reporter gene activity, followed by a long stretch (up to −1052) of enhancer-like sequence and then a sequence upstream of −1052, which appears to contain negative elements. The responses to ABA, ethylene, salt, desiccation and wounding appear to be associated with the −248 bp sequence of the promoter. This region also contains a putative ABRE (CACTGTG) core element. Activation of the osmotin gene by various inducers is discussed in view of antifungal activity of the osmotin protein.
Similar content being viewed by others
References
Andrisani, OM, Pot, DA, Zhu, Z, Dixon, JE: Three sequence-specific DNA-protein complexes are formed with the same promoter element essential for expression of the rat somatostatin gene. Mol Cell Biol 8: 1947–1956 (1988).
Brederode, FT, Linthorst, HJM, Bol, JF: Differential induction of acquired resistance and PR gene expression in tobacco by virus infection, ethephon treatment, UV light and wounding. Plant Mol Biol 17: 1117–1125 (1991).
Broglie, KE, Biddle, P, Cressman, R, Broglie, R: Functional analysis of DNA sequences responsible for ethylene regulation of a bean chitinase gene in transgenic tobacco. Plant Cell 1: 599–607 (1989).
Cordes, S, Deikman, J, Margossian, LJ, Fischer, RL: Interaction of a developmentally regulated DNA-binding factor with sites flanking two different fruit-ripening genes from tomato. Plant Cell 1: 1025–1034 (1989).
Cutt, JR, Klessig, DF: pathogenesis-related proteins. In: Boller, T, Meins, F (eds) Genes Involved in Plant Defense, pp. 209–243. Springer-Verlag, New York (1992).
Datta, N, Cashmore, AR: Binding of a pea nuclear protein to promoters of certain photoregulated genes is modulated by phosphorylation. Plant Cell 1: 1066–1077 (1989).
Deikman, J, Kline, R, Fischer, RL: Organization of ripening and ethylene regulatory regions in a fruit-specific promoter from tomato (Lycopersicon esculentum). Plant Physiol 100: 2013–2017 (1992).
Ecker, JR, Davis, RW: Plant defense genes are regulated by ethylene. Proc Natl Acad Sci USA 84: 5202–5206 (1987).
Goldsbrough, AP, Albrecht, H, Stratford, R: Salicylic acid-inducible binding of a tobacco nuclear protein to a 10 bp sequence which is highly conserved amongst stress-inducible genes. Plant J 3: 563–571 (1993).
Guiltinan, MJ, Marcotte, WRJr, Quatrano, RS: A plant leucine zipper protein that recognizes an abscisic acid response element. Science 250: 267–271 (1990).
Hasegawa, PM, Bressan, RA, Handa, AK: Growth characteristics of a NaCl-selected and non-selected cells of Nicotiana tabacum L. Plant Cell Physiol 21: 1347–1355 (1980).
Jefferson, RA, Kavanagh, TA, Bevan, MW: GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 (1987).
Kononowicz, AK, Nelson, DE, Singh, NK, Hasegawa, PM, Bressan, RA: Regulation of the osmotin gene promoter. Plant Cell 4: 513–524 (1992).
Kononowicz AK, Raghothama KG, Casas AM, Reuveni M, Watad AEA, Liu D, Bressan RA, Hasegawa PM: Osmotin: regulation of gene expression and function. In: Close TJ, Bray EA (eds) Plant Responses to Cellular Dehydration during Environmental Stress. American Society of Plant Physiology, in press (1993).
Lam, E, Benfey, PN, Gilmartin, PM, Rong-Xiang, F, Chua, N-H: Site-specific mutations alter in vitro factor binding and change promoter expression pattern in transgenic plants. Proc Natl Acad Sci USA 86: 7890–7894 (1989).
LaRosa, PC, Chen, Z, Nelson, DE, Singh, NK, Hasegawa, PM, Bressan, RA: Osmotin gene expression is post-transcriptionally regulated. Plant Physiol 100: 409–415 (1992).
Lawton, KA, Raghothama, KG, Goldsbrough, PB, Woodson, WR: Regulation of senescence-related gene expression in carnation flower petals by ethylene. Plant Physiol 93: 1370–1375 (1990).
Lincoln, JE, Cordes, S, Read, E, Fischer, RL: Regulation of gene expression by ethylene during Lycopersicon esculentum (tomato) fruit development. Proc Natl Acad Sci USA 84: 2793–2797 (1987).
Liu D, Raghothama KG, Hasegawa PM, Bressan RA: Resistance to the pathogen Phytophthora infestans in transgenic potato plants that over-express osmotin. Proc Natl Acad Sci USA, in press (1993).
Loake, GJ, Faktor, O, Lamb, CJ, Dixon, RA: Combination of H-box [CCTACC(N)7CT] and G-box (CACGTG) cis elements is necessary for feed-forward stimulation of a chalcone synthase promoter by the phenylpropanoid-pathway intermediate p-coumaric acid. Proc Natl Acad Sci USA 89: 9230–9234 (1992).
Marcotte, WRJr, Russell, SH, Quatrano, RS: Abscisic acid-responsive sequences from the EM gene of wheat. Plant Cell 1: 969–976 (1989).
Miskimins, K, Roberts, MD, McClelland, A, Ruddle, FH: Use of protein-blotting procedure and a specific DNA probe to identify nuclear proteins that recognize the promoter region of the transferring receptor gene. Proc Natl Acad Sci USA 82: 6741–6744 (1985).
Mundy, J, Chua, NH: Abscisic acid and water stress induce the expression of a novel rice gene. EMBO J 7: 2279–2286 (1988).
Mundy, J, Yamaguchi-Shinozaki, K, Chua, N-H: Nuclear proteins bind conserved elements in the abscisic acid-responsive promoter of a rice rab gene. Proc Natl Acad Sci USA 87: 1406–1410 (1990).
Nelson, DE, Raghothama, KG, Singh, NK, Hasegawa, PM, Bressan, RA: Analysis of structure and transcriptional activation of an osmotin gene. Plant Mol Biol 19: 577–588 (1992).
Pla, M, Vilardell, J, Guiltinan, MJ, Marcotte, WR, Niogret, MF, Quatrano, RS, Pages, M: The cis regulatory element CCACGTGG is involved in ABA and water-stress responses of the maize gene rab28. Plant Mol Biol 21: 259–266 (1993).
Quatrano, RS, Guiltinan, MJ, Marcotte, WRJr: Regulation of gene expression by abscisic acid. In Verma, DPS (ed) Control of Plant Gene Expression. CRC Press, Boca Raton, FL, in press (1992).
Raghothama, KG, Lawton, KA, Goldsbrough, PB, Woodson, WR: Characterization of an ethylene-regulated flower senescence-related gene from carnation. Plant Mol Biol 17: 61–71 (1991).
Roberts, WK, Selitrennikoff, CP: Zeamatin, an antifungal protein from maize with membrane permeabilizing activity. J Gen Microbiol 136: 1771–1778 (1990).
Singh, H, Sen, R, Baltimore, D, Sharp, PA: A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature 319: 154–158 (1986).
Singh, NK, LaRosa, PC, Handa, AK, Hasegawa, PM, Bressan, RA: Hormonal regulation of protein synthesis associated with salt tolerance in plant cells. Proc Natl Acad Sci USA 84: 739–743 (1987).
Singh, NK, Nelson, DE, Kuhn, D, Hasegawa, PM, Bressan, RA: Molecular cloning of osmotin and regulation of its expression by ABA and adaptation to low water potential. Plant Physiol 90: 1096–1101 (1989).
Skriver, K, Mundy, J: Gene expression in response to abscisic acid and osmotic stress. Plant Cell 2: 503–521 (1990).
Stintzi, A, Heitz, T, Kaufmann, S, Legrand, M, Fritig, B: Identification of a basic pathogenesis protein of virus-infected tobacco as osmotin. Physiol Mol Plant Path 38: 137–146 (1991).
Terras, FRG, Schoofs, HME, DeBolle, MFC, VanLeuven, F, Rees, SB, Vanderleyden, J, Cammue, BPA, Broekaert, WF: Analysis of two novel classes of plant antifungal proteins from radish (Raphanus sativus L.) seeds. J Biol Chem 267: 15301–15309 (1992).
van derWel, H, Loeve, K: Isolation and characterization of thaumatin I and II, the sweet-tasting proteins from Thaumatococcus daniellii Benth. J Biochem 31: 221–225 (1972).
Vigers, AJ, Roberts, WK, Selitrennikoff, CP: A new family of plant antifungal proteins. Mol Plant-Microbe Interact 4: 315–323 (1991).
Woloshuk, C, Muelenhoff, J, Sela-Buurlage, M, van denElzen, P, Cornelissen, B: Pathogen-induced proteins with inhibitory activity toward Phytophthora infestans. Plant Cell 3: 619–628 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Raghothama, K.G., Liu, D., Nelson, D.E. et al. Analysis of an osmotically regulated pathogenesis-related osmotin gene promoter. Plant Mol Biol 23, 1117–1128 (1993). https://doi.org/10.1007/BF00042346
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00042346