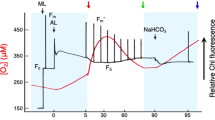
Abstract
We investigated the photodynamic action of hypericin, a natural naphthodianthrone, on photosynthetic electron transport and fluorescence of the cyanobacterium Anacystis nidulans (Synechococcus 6301). The most drastic effect was the inactivation of photosynthetic oxygen evolution in the presence of the electron acceptor phenyl-p-benzoquinone in aerobic cells which required 1 hypericin/5 chlorophyll a for half-maximal effect. Anaerobic A. nidulans was only partially inactivated and variable chlorophyll a fluorescence remained unperturbed suggesting that photoreaction center II was not a target. Further, hypericin, stimulated photoinduced oxygen uptake in the presence of methylviologen in aerobic cells. This action was less specific than the inactivation of oxygen evolution (1 hypericin/0.5–0.7 chlorophyll a for half-maximal effect). Results point to the involvement of molecular oxygen in two ways. Type I mechanism (Henderson BW and Dougherty TJ (1992) Photochem Photobiol 55: 145–157) in which ground state oxygen reacts with excited substrate triplets appears probable for the inactivation of oxygen evolution. On the other hand, Type II mechanism in which excited oxygen singlets react with ground state substrate molecules appears probable in the stimulation of methylviologen mediated oxygen uptake.
Similar content being viewed by others
Abbreviations
- Chl:
-
chlorophyll
- DAD:
-
diaminodurene
- DCMU:
-
3-(3,4-dichlorophenyl)-1,1-dimethyl urea
- Hepes:
-
N-[2-hydroxyethyl]-N′-[ethanesulfonic acid]
- MV:
-
methyl viologen
- PBQ:
-
phenyl-p-benzoquinone
- PPFD:
-
photosynthetic photon flux density
- PS I, PS II:
-
Photosystems I and II
- RC I, RC II:
-
reaction centers of PS I and PS II
References
Arnon DI (1949) Copper enzymes in isolated chloroplasts: Polyphenol oxidase in Beta vulgaris. Plant Physiol 24: 1–15
Castenholz RW (1988) Culturing methods for cyanobacteria. Meth Enzymol 167: 68–93
Cheniae GM and Martin I (1971) Effects of hydroxylamine in Photosystem II. I. Factors affecting the decay of oxygen evolution. Plant Physiol 47: 568–575
Duran N and Song P-S (1986) Hypericin and its photodynamic action. Photochem Photobiol 43: 677–680
Egorov SY, Kamalov VF, Koroteev NI, Krasnovsky AAJr, Toleutaev BN and Zinakov SV (1989) The lifetime of singlet oxygen. Chem Phys Lett 163: 421–424
Gai F, Fehr MJ and Petrich JW (1993) Ultrafast excitation processes in the antiviral agent hypericin. J Am Chem Soc 115: 3384–3385
Giese AC (1971) Photosensitization by natural pigments. In: Giese AC (ed) Photophysiology, Vol 6, pp 77–129. Academic Press, New York
Golbeck JH and Bryant DA (1991) Photosystem I. In: Lee CP (ed) Current Topics in Bioenergetics, Vol 16, pp 83–177. Academic Press, San Diego
Govindjee (1990) Photosystem II heterogeneity: The acceptor side. Photosynth Res 25: 151–160
Govindjee (1995) Sixity-three years since Kautsky: Chlorophyll a fluorescence. Aust J Plant Physiol 22: 131–160
Hadjur C, Jeunet A and Jardon P (1994) Photosensitization by hypericin: Electron spin (ESR) evidence for the formation of singlet oxygen and superoxide anion radicals in an in vitro model. J Photochem Photobiol B: Biol 26: 67–74
Henderson BW and Dougherty TJ (1992) How does photodynamic therapy work? Photochem Photobiol 55: 145–157
Iwaki M and Itoh S (1989) Electron transfer in spinach Photosystem I reaction center containing benzo-naphtho-and anthraquinones in place of phylloquinone. FEBS Lett 256: 11–16
Jardon P, Lazortchak N and Gautron R (1987) Formation d'oxygène singulet 1Δg photosensibilisée par l'hypericine. Caracterisation et étude du mechanisme par spectroscopie laser. J Chim Phys 84: 1143–1145
Joshi MK and Mohanty P (1995) Probing photosynthetic performance by chlorophyll a fluorescence: Analysis and interpretation of fluorescence parameters. J Sci Ind Res 54: 155
Krause GH and Weis E (1991) Chlorophyll fluorescence and photosynthesis: The basics. Ann Rev Plant Physiol Plant Mol Biol 42: 313–349
Lenard J, Rabson A and Vanderoef R (1993) Photodynamic inactivation of infectivity of human immunodeficiency virus and other enveloped viruses using hypericin and rose bengal: Inhibition of fusion and syncytia. Proc Natl Acad Sci USA 90: 158–162
Liebes L, Mazur Y, Freeman D, Lavie G, Kudler N, Mendoza S, Levin B, Hochster H and Meruelo D (1991) A method for the quantitation of hypericin, an antiviral agent, in biological fluids using high performance liquid chromatography. Anal Biochem 195: 77–85
Malkin J and Mazur Y (1993) Hypericin derived triplet states and transients in alcohols and water. Photochem Photobiol 57: 929–933
Meruelo D, Lavie G and Lavie D (1988) Therapeutic agents with dramatic antiretroviral activity and little toxicity at effective doses: Aromatic polycyclic diones hypericin and pseudohypericin. Proc Natl Acad Sci USA 85: 5230–5234
Mullineaux CW and Allen JF (1988) Fluorescence induction transients indicate dissociation of Photosystem II from the phycobilisome during the state-2 transition in the cyanobacterium Synechococcus 6301. Biochim Biophys Acta 934: 96–10
Mullineaux CW, Bittersmann E, Allen JF and Holzwarth AR (1990) Picosecond time-resolved fluorescence emission spectra indicate decreased energy transfer from the phycobilisome to Photosystem II in light-state 2 in the cyanobacterium Synechococcus 6301. Biochim Biophys Acta 1015: 231–242
Myers J, Graham JR and Wang RT (1980) Light harvesting in Anacyctis nidulans studied in pigment mutants. Plant Physiol 66: 1144–1149
Pace N (1942) The etiology of hypericism, a photosensitivity produced by St. Johnswort. Am J Physiol 136: 650–656
Papageorgiou GC (1975) Chlorophyll fluorescence: An intrinsic probe of photosynthesis. In: Govindjee (ed) Bioenergetics of Photosynthesis, pp 319–371. Academic Press, New York
Ratajczak R, Mitchell R and Haehnel W (1988) Properties of the oxidizing side of Photosystem I. Biochim Biophys Acta 933: 306–318
Renger G, Hanssum B, Gleiter H, Koike H and Inoue Y (1988) Interaction of 1, 4 benzoquinones with Photosystem II in the thylakoids and Photosystem II membrane fragments from spinach. Biochim Biophys Acta 936: 435–446
Samuelson G, Lonneborg A, Rosenqvist E, Gustaffson P and Oquist G (1985) Photoinhibition and reactivation of photosynthesis in the cyanobacterium Anacystis nidulans. Plant Physiol 79: 992–995
Stevens SEJr. Patterson COP and Myers J (1973) The production of hydrogen peroxide by blue-green algae: A survey. J Phycol 9: 427–430
Tytler EM, Whitelam GC, Hipkins MF and Codd GA (1984) Photoinactivation of Photosystem II during photoinhibition of the cyanobacterium Microcystis aeruginosa. Planta 160: 229–234
Vonshak A, Guy R, Poplawsky R and Ohad I (1988) Photoinhibition and its recovery in two strains of the cyanobacterium Spirulina platensis. Plant Cell Physiol 29: 721–726
Yocum CF, Yerkes CT, Blankenship RE, Sharp RR and Babcock GT (1981) Stoichiometry, inhibitor sensitivity and organization of manganese associated with photosynthetic oxygen evolution. Proc Natl Acad Sci USA 78: 7507–7511
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Papageorgiou, G.C., Alygizaki-Zorba, A., Loukas, S. et al. Photodynamic effects of hypericin on photosynthetic electron transport and fluorescence of Anacystis nidulans (Synechococcus 6301). Photosynth Res 48, 221–226 (1996). https://doi.org/10.1007/BF00041012
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00041012