Summary
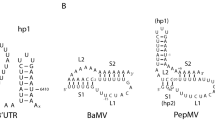
The nucleotide sequences of viroids contain features believed to be essential for the splicing of group I introns. Common sequence elements include a 16-nucleotide consensus sequence and three pairs of short sequences arranged in the same sequential order in both types of RNAs. The calculated probability of finding sequences resembling the 16-nucleotide consensus sequence in random nucleotide chains showed that at low fidelity (up to 5 mismatched nucleotides), the number of such sequences in viroids, plant viral satellite RNAs, plant viral RNAs and one plant viral DNA, group I introns and flanking exons does not significantly differ from the number expected at random. As the degree of fidelity is increased, the number in both introns and viroids, but not in exons or the other plant pathogens examined, greatly exceeds that expected in random chains. These findings suggest that viroids may have evolved from group I introns and/or that processing of viroid oligomers to monomers may have structural requirements similar to those of group I introns. The nucleotide sequences of viroids do not show close homology with two conserved regions of group II introns, the 14-base pair consensus region and the 5′ terminal segment. However, close homology does exist between the conserved sequence of the 3′ terminal segment of group II introns and viroids thus suggesting a possible evolutionary or functional relationship.
Similar content being viewed by others
References
Ahlquist P, Lukcow V, Kaesberg P: Complete nucleotide sequence of brome mosaic virus RNA3. J Mol Biol 153:23–38, 1981.
Arnberg AC, van der Horst G, Tabak HF: Formation of lariats and circles in self-splicing of the precursor to the large ribosomal RNA of yeast mitochondria. Cell 44:235–242, 1986.
Bonitz SA, Coruzzi G, Thalenfeld BE, Tzagoloff A, Macino G: Assembly of the mitochondrial membrane system. J Biol Chem 255:11927–11941, 1980.
Branch AD, Robertson HD: A replication cycle for viroids and other small infectious RNAs. Science 223:450–455, 1984.
Burke JM, Irvine KD, Kaneko KJ, Kerker BJ, Oettgen AB, Tierney WM, Williamson CL, Zaug AJ, Cech TR: Role of conserved sequence elements 9L and 2 in self-splicing of the Tetrahymena ribosomal RNA precursor. Cell 45:167–176, 1986.
Burke JM, RajBhandary UL: Intron within the large rRNA gene of N. crassa mitochondria: a long open reading frame and consensus sequence possibly important in splicing. Cell 31:509–520, 1982.
Buzayan JM, Gerlach WL, Bruening G, Keese P, Gould AR: Nucleotide sequence of satellite tobacco ringspot virus RNA and its relationship to multimeric forms. Virology 151:186–199, 1986.
Cech TR: The generality of self-splicing RNA: relationship to nuclear mRNA splicing. Cell 44:207–210, 1986.
Cech TR, Tanner NK, Tinoco Jr IT, Weir BR, Zuker M, Perlman PS: Secondary structure of the Tetrahymena ribosomal RNA intervening sequence: structural homology with fungal mitochondrial intervening sequences. Proc Natl Acad Sci USA 80:3903–3907, 1983.
Collmer CW, Hadidi A, Kaper JM: Nucleotide sequence of the satellite of peanut stunt virus reveals structural homologies with viroids and certain nuclear and mitochondrial introns. Proc Natl Acad Sci USA 82:3110–3114, 1985.
Crick F: Split genes and RNA splicing. Science 204:264–271, 1979.
Davies RW, Waring RB, Ray JA, Brown TA, Scazzocchio C: Making ends meet: a model for RNA splicing in fungal mitochondria. Nature (London) 300:719–724, 1982.
De La Salle H, Jacq C, Slonimski PP: Critical sequences within mitochondrial introns: pleiotropic mRNA maturase and cis-dominant signals of the box intron controlling reductase and oxidase. Cell 28:721–732, 1982.
Diener TO: Viroids: Structure and function. Science 205:859–866, 1979.
Diener TO: Are viroids escaped introns? Proc Natl Acad Sci USA 78:5014–5015, 1981.
Diener TO: Viroids. Adv Virus Res 28:241–283, 1983.
Diener TO: Viroid processing: a model involving the central conserved region and hairpin I. Proc Natl Acad Sci USA 82:58–62, 1986.
Diener TO, Hadidi A: Viroids: The intron connection. Microbiologica (in press), 1986.
Dinter-Gottlieb G: Viroids and virusoids are related to group I introns. In: Diener TO (ed). The Viroids. Plenum Press, New York, (in press), 1986.
Fox DT, Leaver CJ: The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codons. Cell 26:315–323, 1981.
Franck A, Jonard G, Richards K, Hirth L, Guiley H: Nucleotide sequence of cauliflower mosaic virus DNA. Cell 21:285–294, 1980.
Garriga G, Lambowitz AM: RNA splicing in Neurospora mitochondria: self-splicing of a mitochondrial intron in vitro. Cell 39:631–641, 1984.
Goelet GP, Lomonssoff PJG, Butler ME, Akam M, Gait J, Karn J: Nucleotide sequence of tobacco mosaic virus. Proc Natl Acad Sci USA 79:5818–5822, 1982.
Gross HJ, Domdey H, Lossow C, Jank P, Raba M, Alberty H, Sänger HL: Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature (London) 273:203–208, 1978.
Gross HJ, Krupp G, Domdey H, Raba M, Jank P, Lossow C, Alberty H, Ramm K, Sänger HL: Nucleotide sequence and secondary structure of citrus exocortis and chrysanthemum stunt viroid. Eur J Biochem 121:249–257, 1982.
Hallreich A, Pajot P, Foucher M, Grandchamp C, Slonimski P: A pathway of cytochrome b mRNA processing in yeast mitochondria: specific splicing steps and an intronderived circular RNA. Cell 19:321–329, 1980.
Haseloff J, Mohamed NA, Symons RH: Viroid RNAs of the cadang cadang disease of coconut. Nature (London) 299:316–321, 1982.
Haseloff J, Symons RH: Chrysanthemum stunt viroid-primary sequence and secondary structure. Nucl Acids Res 9:2741–2752, 1981.
Haseloff J, Symons RH: Comparative sequence and structure of viroid-like RNAs of two plant viruses. Nucl Acids Res 10:3681–3691, 1982.
Hensgens LAM, Arnberg AC, Roosendaal E, van der Horst G, Van der Veen R, Van Ommen GJB, Grivell LA: Variation, transcription, and circular RNAs of the mitochondrial gene for subunit 1 of cytochrome c oxidase. J Mol Biol 164:35–38, 1982.
Hensgens LAM, Bonen L, deHaan M, Van der Horst G, Grivell LA: Two intron sequences in yeast mitochondrial COX1 gene: Homology among URF-containing introns and strain dependent variation in flanking exons. Cell 32:379–389, 1983.
Keese P, Bruening G, Symons RH: Comparative sequence and structure of circular RNAs from two isolates of lucerne transient streak virus. FEBS Lett 159:185–190, 1983.
Keese P, Symons RH: Domains in Viroids: Evidence of intermolecular RNA rearrangements and their contribution to viroid evolution. Proc Natl Acad Sci USA 82:4582–5486, 1985.
Keller M, Michel F: The introns of the Euglenia gracilis chloroplast gene which codes for the 32-KDa protein of photosystem II. FEBS Lett 179:69–73, 1985.
Kiefer MC, Owens RA, Diener TO: Structural similarities between viroids and transposable genetic elements. Proc Natl Acad Sci USA 80:6234–6238, 1983.
Kikuchi Y, Tyc K, Filipowicz W, Sänger HL, Gross HJ: Circularization of linear viroid RNA via 2′-phosphomonoester, 3′,5′-phosphodiester bonds by a novel type of RNA ligase from wheat germ and Chlamydomonas. Nucl Acids Res 10:7521–7529, 1982.
Michel F, Dujon B: Conservation of RNA secondary structures in two intron families including mitochondrial-, chloroplast-, and nuclear-encoded members. EMBO J 2:33–38, 1983.
Michel F, Jacquier A, Dujon B: Comparison of fungal mitochondrial introns reveals extensive homologies in RNA secondary structure. Biochimie 64:867–881, 1982.
Michel F, Lang BF: Mitochondrial class II introns encode proteins related to the reverse transcriptases of retroviruses. Nature 316:641–643, 1985.
Nargang FE, Bell JB, Stohl LL, Lambowitz AM: The DNA sequence and genetic organization of a Neurospora mitochondrial plasmid suggest a relationship to introns and mobile elements. Cell 38:441–453, 1984.
Netter P, Jacq C, Carignani G, Slonimski PP: Critical sequence within mitochondrial introns: cis-dominant mutations of the ‘cytochrome-b-like’ intron of the oxidase gene. Cell 28:733–738, 1982.
Nobrega FG, Tzagoloff A: Assembly of the mitochondrial membrane system. J Biol Chem 255:9828–9837, 1980.
Noller HF, Woese CR: Secondary structure of 16S ribosomal RNA. Science 212:403–410, 1981.
Nomiyama H, Kuhara S, Kukita T, Otsuka T, Sakaki Y: Nucleotide sequence of the ribosomal RNA gene of Physarum polycephalum: intron 2 and its flanking regions of the 26S rRNA gene. Nucl Acids Res 9:5507–5520, 1981.
Ohno T, Takamatsu N, Meshi T, Okada Y: Hop stunt viroid: molecular cloning and nucleotide sequence of the complete cDNA copy. Nucl Acids Res 11:6185–6197, 1983.
Palukatis P, Zaitlin M: The nature and biological significance of linear potato spindle tuber viroid molecules. In Robertson HD, Howell SH, Zaitlin M, Malmberg RL (eds) Plant Infectious Agents-Viruses, Viroids, Virusoids, and Satellites. Cold Spring Harbor Laboratory, New York, 1983, pp 136–140.
Peebles CL, Perlman PS, Mecklenburg KL, Petrillo ML, Tabor JH, Jarrell KA, Cheng H-L: A self-splicing RNA excises an intron larriat. Cell 44:213–223, 1986.
Prody GA, Bakos JT, Buzayan JM, Schneider IR, Bruening G: Autolytic processing of dimeric plant virus satellite RNA. Science 231:1577–1580, 1986.
Richards KE, Jonard G, Jacquemond M, Lott H: Nucleotide sequence of cucumber mosaic virus associated RNA 5. Virology 89:395–408, 1978.
Robertson HD, Rosen DL, Branch AD: Cell-free synthesis and processing of an infectious dimeric transcript of potato spindle tuber viroid RNA. Virology 142:441–447, 1985.
Sänger HL: Nucleic acids and proteins in Plants II, Vol 14B. In Parthier B, Boultes D (eds). Encyclopedia of Plant Physiology, New Series. Springer-Verlag. Berlin/Heidelberg, 1982, pp 368–454.
Sano T, Uyeda I, Shikata E, Ohno T, Okada Y: Nucleotide sequence of cucumber pale fruit viroid: homology to hop stunt viroid. Nucl Acids Res 12:3427–3434, 1984.
Symons RH: Avocado sunblotch viroid: Primary sequence and proposed secondary structure. Nucl Acids Res 9:6527–6537, 1981.
Tabak HF, Van der Horst G, Osinga KA, Arnberg AC: Splicing of large ribosomal precursor RNA and processing of intron RNA in yeast mitochondria. Cell 39:623–629, 1984.
van der Horst G, Tabak HF: Self-splicing of yeast mitochondrial ribosomal and messenger RNA precursors. Cell 40:759–766, 1985.
van der Veen R, Arnberg AC, van der Horst G, Bonen L, Tabak HF, Grivell LA. Excised Group II introns in yeast mitochondria are lariats and can be formed by self-splicing in vitro. Cell 44:225–234, 1986.
Visvader JE, Gould AR, Bruening GE, Symons RH: Citrus exocortis viroid: Nucleotide sequence and secondary structure of an Australian isolate. FEBS Lett 137: 288–292, 1982.
Visvader JE, Forster AC, Symons RH: Infectivity and in vitro mutagenesis of monomeric cDNA clones of citrus exocortis viroid indicates the site of processing of viroid precursors. Nucl Acids Res 13:5843–5856, 1985.
Waring RB, Davies RW: Assessment of a model for intron RNA secondary structure relevant to RNA-self-splicing-a review. Gene 23:227–291, 1984.
Waring RB, Davies RW, Lee S, Grisi E, Berks MM, Scazzocchio C: The mosaic organization of the apocytochrome b gene of Aspergillus nidulans revealed by DNA sequencing. Cell 27: 4–11, 1981.
Waring RB, Davies RW, Scazzocchio C, Brown TA: Internal structure of mitochondrial intron of Aspergillus nidulans. Proc Natl Acad Sci USA 79:6332–6336, 1982.
Waring RB, Scazzocchio C, Brown TA, Davies RW: Close relationship between certain nuclear and mitochondrial introns. J Mol Biol 167: 595–605, 1983.
Weiss-Brummer B, Holl J, Schweyen RJ, Rodel G, Kaudewitz F: Processing of yeast mitochondrial RNA: Involvement of intermolecular hybrids in splicing of cob intron 4 RNA by mutation and reversion. Cell 33: 195–202, 1983.
Wild MA, Sommer R: Sequence of a ribosomal RNA gene intron from Tetrahymena. Nature (London) 283: 693–694, 1980.
Wolf P, Gilz R, Schumacher J, Riesner D: Complexes of viroids with histones and other proteins. Nucl Acids Res 13: 355–367, 1985.
Zaug AJ, Gabrowski PJ, Cech TR: Autocatalytic cyclization of an excised intervening sequence is a cleavageligation reaction. Nature 301: 578–583, 1983.
Zimmern D: Homologies proteins encoded by yeast mitochondrial introns and by a group of RNA viruses from plants. J Mol Biol 171: 345–352, 1983.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hadidi, A. Relationship of viroids and certain other plant pathogenic nucleic acids to group I and II introns. Plant Mol Biol 7, 129–142 (1986). https://doi.org/10.1007/BF00040139
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00040139