Abstract
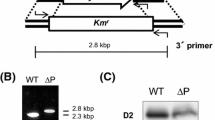
Photosystem I catalyzes the light-driven oxidation of plastocyanin or cytochrome c 6 and the reduction of ferredoxin or flavodoxin. PsaJ is a 4.4 kDa hydrophobic subunit of photosystem I from cyanobacteria and chloroplasts. To investigate the function of PsaJ, we generated a mutant strain of the cyanobacterium Synechocystis sp. PCC 6803 in which the psaJ gene is replaced by a gene for chloramphenicol resistance. Deletion of psaJ led to a reduction in the steady state RNA level from psaF which is located upstream from psaJ. Immunoquantification using an anti-PsaF antibody revealed a significant decrease in the amount of PsaF in membranes of the mutant strain. Trimeric photosystem I complexes isolated from the mutant strain using n-dodecyl β-D-maltoside lacked PsaJ, contained ca. 80% less PsaF, but maintained wild-type levels of other photosystem I subunits. In contrast, the photosystem I purified using Triton X-100 contained less than 2% PsaF when compared to the wild type, showing the more extractable nature of PsaF in PsaJ-less photosystem I in the presence of Triton X-100. PsaE was more accessible to removal by NaI in a mutant strain lacking PsaF and PsaJ than in the wild type. The presence of PsaF in photosystem I from the PsaJ-less strain did not alter the increased susceptibility of PsaE to removal by NaI. These results indicate an interaction between PsaJ and PsaF in the organization of the complex.
Similar content being viewed by others
References
Arnon D: Copper enzyme in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol 24: 1–14 (1949).
Bryant D: Molecular biology of Photosystem I. In: Barber J (ed) The Photosystems: Structure, Function and Molecular Biology, pp. 501–549. Elsevier, Amsterdam (1992).
Chitnis PR, Nelson N: Photosystem I. In: Bogorad L, Vasil IK (eds) The Photosynthetic Apparatus: Molecular Biology and Operation, pp. 178–224. Academic Press, San Diego (1991).
Chitnis PR, Nelson N: Assembly of two subunits of the cyanobacterial photosystem I on the n-side of thylakoid membranes. Plant Physiol 99: 239–246 (1992).
Chitnis PR, Purvis D, Nelson N: Molecular cloning and targeted mutagenesis of the gene psaF encoding subunit III of photosystem I from the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem 266: 20146–20151 (1991).
Chitnis PR, Reilly PA, Miedel MC, Nelson N: Structure and targeted mutagenesis of the gene encoding 8-kDa subunit of photosystem I of the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem 264: 18374–18380 (1989).
Chitnis PR, Reilly PA, Nelson N: Insertional inactivation of the gene encoding subunit II of photosystem I of the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem 264: 18381–18385 (1989).
Chitnis VP, Chitnis PR: PsaL subunit is required for the formation of photosystem I trimers in the cyanobacterium Synechocystis sp. 6803. FEBS Lett 336: 330–334 (1993).
Chitnis VP, Xu Q, Yu L, Golbeck JH, Nakamoto H, Xie D-L, Chitnis PR: Targeted inactivation of the gene psaL encoding a subunit of photosystem I of the cyanobacterium Synechocystis sp. 6803. J Biol Chem 268: 11678–11684 (1993).
Enami I, Ohta H, Katoh S: Topological studies on subunit polypeptides of the PS I reaction center complex in the thylakoid membranes of the thermophilic cyanobacterium Synechococcus sp. Plant Cell Physiol 27: 1395–1405 (1986).
Glazer AN, Melis A: Photochemical reaction centers: structure, organization and function. Annu Rev Plant Physiol 38: 11–45 (1987).
Golbeck JH: Shared thematic elements in photochemical reaction centers. Proc Natl Acad Sci USA 90: 1642–1646 (1993).
Hippler M, Ratajczak R, Haehnel W: Identification of the plastocyanin binding subunit of photosystem I. FEBS Lett 250: 280–284 (1989).
Ikeuchi M, Hirano A, Hiyama T, Inoue Y: Polypeptide composition of higher plant photosystem I complex: Identification of psaI, psaJ and psaK gene products. FEBS Lett 263: 274–278 (1990).
Ikeuchi M, Nyhus KJ, Inoue Y, Pakrasi HB: Identities of four low-molecular-mass subunits of the photosystem I complex from Anabaena variabilis ATCC 29413: evidence for the presence of the psaI gene product in a cyanobacterial complex. FEBS Lett 287: 5–9 (1991).
Ikeuchi M, Sonoike K, Koike H, Pakrasi H, Inoue Y: A novel 3.5 kDa protein component of cyanobacterial photosystem I complexes. Plant Cell Physiol 33: 1057–1063 (1993).
Kjærulff S, Andersen B, Nielsen VS, Møller BL, Okkels JS: The PSI-K Subunit of Photosystem I from Barley (Hordeum vulgare L.): Evidence for a gene duplication of an ancestral PSI-G/k gene. J Biol Chem 268: 18912–18916 (1993).
Krauss N, Hinrichs W, Witt I, Fromme P, Pritzkow W, Dauter Z, Betzel C, Wilson KS, Witt HT, Saenger W: Three-dimensional structure of system I of photosynthesis at 6 Å resolution. Nature 361: 326–331 (1993).
Kruip J, Boekema EJ, Bald D, Boonstra AF, Rogner M: Isolation and structural Characterization of monomeric and trimeric photosystem I complexes (P700-FA/FB and P700-FX) from the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem 268: 23353–23360 (1993).
Li N, Zhao J, Warren PV, Warden JT, Bryant DA, Golbeck JH: PsaD is required for the stable binding of PsaC to the photosystem I core protein of Synechococcus sp. PCC 6301. Biochemistry 30: 7863–7872 (1991).
Mühlenhoff U, Haehnel W, Witt HT, Herrmann RG: Genes encoding eleven subunits of photosystem I from the thermophilic cyanobacterium synechococcus sp. Gene 127: 71–78 (1993).
Oh-oka H, Takahashi Y, Kuriyama K, Saeki K, Matsubara H: The protein responsible for center A/B in spinach photosystem I: isolation with iron sulfur cluster(s) and complete sequence analysis. J Biochem 103: 962–968 (1988).
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY: Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111: 1–61 (1979).
Rousseau F, Setif P, Lagoutte B: Evidence for the involvement of PSI-E subunit in the reduction of ferredoxin by photosystem I. EMBO J 12: 1755–1765 (1993).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY (1989).
Scheller HV, Okkels JS, Høj PB, Svendsen I, Roepstorff P, Møller BL: The primary structure of a 4.0-kDa photosystem I polypeptide encoded by the chloroplast psaI gene. J Biol Chem 264: 18402–18406 (1989).
Sonoike K, Hatanaka H, Katoh S: Small subunits of photosystem I reaction center complexes from Synechococcus elongatus. II. The psaE gene product has a role to promote interaction between the terminal electron acceptor and ferredoxin. Biochim Biophys Acta 1141: 52–57 (1993).
Strotmann H, Weber N: On the function of PsaE in chloroplast photosystem I. Biochim Biophys Acta 1143: 204–210 (1993).
Vieira J, Messing J: The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19: 259–268 (1982).
Williams JGK: Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Meth Enzymol 167: 766–778 (1988).
Wynn R, Omaha J, Malkin R: Structural and functional properties of the cyanobacterial photosystem I complex. Biochemistry 28: 5554–5560 (1989).
Wynn RM, Luong C, Malkin R: Maize photosystem. I. Identification of the subunit which binds plastocyanin. Plant Physiol 91: 445–449 (1989).
Wynn RM, Malkin R: Interaction of plastocyanin with photosystem I: a chemical cross-linking study of the polypeptide that binds to plastocyanin. Biochemistry 27: 5863–5869 (1988).
Xu Q, Jung YS, Chitnis VP, Guikema JA, Golbeck JH, Chitnis PR: Mutational analysis of photosystem I polypeptides in Synechocystis sp. PCC6803: Subunit requirements for reduction of NADP+ mediated by ferredoxin and flavodoxin. J Biol Chem, 269: 215–218 (1994).
Xu Q, Yu L, Chitnis VP, Chitnis PR: Function and organization of photosystem I in a cyanobacterial mutant strain that lacks PsaF and PsaJ subunits. J Biol Chem 269: 3205–3211 (1994).
Yu L, Zhao J, Mühlenhoff U, Bryant DA, Golbeck JH: PsaE is required for in vivo cyclic electron flow around photosystem I in the cyanobacterium Synechococcus sp. PCC 7002. Plant Physiol 103: 171–180 (1993).
Zanetti G, Merati G: Interaction between photosystem I and ferredoxin: identification by chemical cross-linking of the polypeptide which binds ferredoxin. Eur J Biochem 169: 143–146 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Xu, Q., Odom, W.R., Guikema, J.A. et al. Targeted deletion of psaJ from the cyanobacterium Synechocystis sp. PCC 6803 indicates structural interactions between the PsaJ and PsaF subunits of photosystem I. Plant Mol Biol 26, 291–302 (1994). https://doi.org/10.1007/BF00039540
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00039540