Abstract
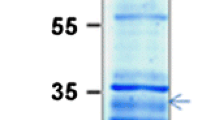
The recently cloned cDNA for pea chloroplast thioredoxin f was used to produce, by PCR, a fragment coding for a protein lacking the transit peptide. This cDNA fragment was subcloned into a pET expression vector and used to transform E. coli cells. After induction with IPTG the transformed cells produce the protein, mainly in the soluble fraction of the broken cells. The recombinant thioredoxin f has been purified and used to raise antibodies and analysed for activity. The antibodies appear to be specific towards thioredoxin f and do not recognize other types of thioredoxin. The recombinant protein could activate two chloroplastic enzymes, namely NADP-dependent malate dehydrogenase (NADP-MDH) and fructose 1,6-bisphosphatase (FBPase), both using dithiothreitol as a chemical reductant and in a light-reconstituted/thylakoid assay. Recombinant pea thioredoxin f turned out to be an excellent catalyst for NADP-MDH activation, being the more efficient than a recombinant m-type thioredoxin of Chlamydomonas reinhardtii and the thioredoxin of E. coli. At the concentrations of thioredoxin used in the target enzyme activation assays only the recombinant thioredoxin f activated the FBPase.
Similar content being viewed by others
References
Aguilar F, Brunner B, Gardet-Salvi L, Stutz E, Schürmann P: Biosynthesis of active spinach-chloroplast thioredoxin f in transformed E. coli. Plant Mol Biol 20: 301–306 (1992).
Bakrim N, Echevarria C, Crétin C, Arrio-Dupont M, Pierre J-N, Vidal J, Chollet R, Gadal P: Regulatory phosphorylation of Sorghum leaf phosphoenolpyruvate carboxylase. Identification of the protein-serine kinase and some elements of the signal-transduction cascade. Eur J Biochem 204: 821–830 (1990).
Buc J, Rivière M, Gontero B, Sauve P, Meunier J-C, Ricard J: Affinity chromatography, on fructose-bisphosphatase-sepharose of two chloroplastic thioredoxin f. Purification and comparative molecular properties. Eur J Biochem 140: 199–202 (1984).
Buchanan BB: Role of light in the regulation of chloroplast enzymes. Annu Rev Plant Physiol 31: 341–374 (1980).
Buchanan BB: Regulation of CO2 assimilation in oxygenic photosynthesis: the ferredoxin/thioredoxin system. Arch Biochem Biophys 288: 1–9 (1991).
Cséke C, Buchanan BB: Regulation of the formation and utilization of photosynthate in leaves. Biochim Biophys Acta 853: 43–63 (1986).
Decottignies P, Schmitter J-M, Jacquot J-P, Dutka S, Picaud A, Gadal P: Purification, characterization, and complete amino acid sequence of a thioredoxin from a green algae, Chlamydomonas reinhardtii. Arch Biochem Biophys 280: 112–121 (1990).
De Lamotte-Guery F, Miginiac-Maslow M, Decotignies P, Stein M, Minard P, Jacquot J-P: Mutation of a negatively charged amino acid in thioredoxin modifies its reactivity with chloroplastic enzymes. Eur J Biochem 196: 287–294 (1991).
Droux M, Miginiac-Maslow M, Jacquot J-P, Gadal P, Crawford NA, Kosower NS, Buchanan BB: Ferredoxin-thioredoxin reductase: a catalytically active dithiol group links photoreduced ferredoxin to thioredoxin functional in photosynthetic enzyme regulation. Arch Biochem Biophys 256: 372–380 (1987).
Florencio FJ, Yee BC, Johnson TC, Buchanan B: An NADP/thioredoxin system in leaves: purification and characterization of NADP-thioredoxin reductase and thioredoxin h from spinach. Arch Biochem Biophys 266: 496–507 (1988).
Hirel Ph H, Schmitter J-M, Dessen P, Fayat G, Blanquet S: Extent of N-terminal methionine excision from E. coli proteins is governed by the side-chain length of the penultimate amino acid. Proc Natl Acad Sci (USA) 86: 8247–8251 (1989).
Huppe HC, de Lamotte-Guéry F, Jacquot J-P, Buchanan BB: The ferredoxin-thioredoxin system of a green algae, Chlamydomonas reinhardtii. Planta 180: 341–351 (1990).
Issakidis E, Miginiac-Maslow M, Decottiginies P, Jacquot J-P, Crétin C, Gadal P: (1992) Site-directed mutagenesis reveals the involvement of an additional thioredoxin-dependent regulatory site in the activation of recombinant sorghum leaf NADP-malate dehydrogenase. J Biol Chem 267: 21577–21583 (1992).
Jacquot J-P, Buchanan BB, Martin F, Vidal J: Enzyme regulation in C4 photosynthesis. Plant Physiol 68: 300–304 (1981).
Jacquot J-P, Vidal J, Gadal P, Schürmann P: Evidence for the existence of several enzyme specific thioredoxins in plants. FEBS Lett 96: 243–246 (1978).
Kamo M, Tsugita A, Wiessner C, Wedel N, Bartling D, Herrmann RG, Aguilar F, Gardet-Salvi L, Schürmann P: Primary structure of spinach-chloroplast thioredoxin f. Protein sequencing and analysis of complete cDNA clones for spinach-chloroplast thioredoxin f. Eur J Biochem 182: 315–322 (1989).
Le Maréchal P, Hoang BMC, Schmitter J-M, Van Dorsselaer A, Decottignies P: Purification, properties and primary structure of thioredoxin from Aspergillus nidulans. Eur J Biochem 210: 421–429 (1992).
Lepiniec L, Hodges M, Gadal P, Crétin C: Isolation, characterization and nuclebtide sequence of a full-length pea cDNA encoding thioredoxin-f. Plant Mol Biol 18: 1023–1025 (1992).
Nishizawa AN, Buchanan BB: Enzyme regulation in C4 photosynthesis. Purification and properties of thioredoxin-linked fructose bisphosphatase and sedoheptulose bisphosphatase from corn leaves. J Biol Chem 256: 6119–6126 (1981).
Prado FE, Lazaro JJ, Hermoso R, Chueca A, Lopez-Gorgé J: Purification and properties of pea (Pisum sativum L.) thioredoxin f, a plant thioredoxin with unique features in the activation of chloroplast fructose-1,6-bisphosphatase. Planta 188: 345–353 (1992).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Schägger H, von Jagow G: Tricine-sodium dodecyl sulfate polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166: 368–379 (1987).
Schürmann P, Jacquot J-P: Improved in vitro light activation and assay systems for two spinach chloroplast enzymes. Biochim Biophys Acta 569: 309–312 (1979).
Schürmann P, Maeda K, Tsugita A: Isomers in thioredoxins of spinach chloroplasts. Eur J Biochem 116: 37–45 (1981).
Tsugita A, Maeda K, Schürmann P: Spinach chloroplast thioredoxins in evolutionary drift. Biochem Biophys Res Comm 115: 1–7 (1983).
Vidal J, Godbillon G, Gadal P: Recovery of active highly purified phosphoenolpyruvate carboxylase from specific immunoabsorbent column. FEBS Lett 118: 31–34 (1980).
Whittaker MM, Gleason FK: Isolation and characterization of thioredoxin f from the filamentous cyanobacterium, Anabaena sp. 7119. J Biol Chem 259: 14088–14093 (1984).
Wolosiuk RA, Crawford NA, Yee BC, Buchanan BB: Isolation of three thioredoxins from spinach leaves. J Biol Chem 254: 1627–1632 (1979).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hodges, M., Miginiac-Maslow, M., Decottignies, P. et al. Purification and characterization of pea thioredoxin f expressed in Escherichia coli . Plant Mol Biol 26, 225–234 (1994). https://doi.org/10.1007/BF00039534
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00039534