Abstract
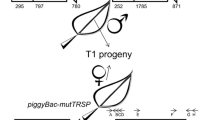
A Tam3 two-element system has been designed by combining an immobilized Tam3 element with a non-autonomous dTam3 element inserted into the HPT gene. The phenotypic assay employed, restored hygromycin resistance, indicated thattrans-activation of the non-autonomous dTam3 element occurred. Molecular analyses of the excision sites revealed that the ends of the dTam3 element remain in the empty donor sites. The predominant consequence of this type of excision appears to be that excised fragments fail to re-integrate into the tobacco genome. Only one case of dTam3 re-integration could be detected. The ends of this element had been degraded upon integration into the tobacco genome. Either the altered structure of the Tam3 derivatives or tobacco host factors are influencing thetrans-activation of a dTam3 element, resulting in aberrant excision.
Similar content being viewed by others
References
Baker B, Coupland G, Federoff N, Starlinger P, Schell J: Phenotypic assay for excision of the maize controlling elementAc in tobacco. EMBO J: 154-1554 (dy1987).
Coen ES, Carpentier R, Martin C: Transposable elements generate novel spatial patterns of gene expression inAntirrhinum majus. Cell 47: 285–296 (1986).
Coen ES, Robbins TP, Almeida J, Hudson A, Carpentier R: Consequences and mechanisms of transposition inAntirrhinum majus. In: Berg DE, Howe MM (eds) Mobile DNA, p. 413–437. American Society for Microbiology (1989).
Dower WJ, Miller JF, Ragsdale CW: High efficiency transformation ofE. coli by high voltage electroporation. Nucl Acids Res 16: 6127–6145 (1988).
Engels WR, Johnson-Schiltz DM, Eggleston WB, Sved J: High-frequency P-element loss inDrosophila is homolog dependent. Cell 62: 515–525 (1990).
Federoff N: Maize transposable elements. In: Berg DE, Howe MM (eds) Mobile DNA, pp. 375-411. American Society for Microbiology (1989).
Fedoroff N: About maize transposable elements and development. Cell 56: 18L191 (1989).
Gierl A, Lutticke S, Saedler H: TnpA product encoded by the transposable element En-1 ofZea mays is a DNA binding protein. EMBO J 7: 4045–4053 (1988).
Greenblatt IM: A chromosome replication pattern deduced from pericarp phenotypes resulting from movements of the transposable element, Modulator, in maize. Genetics 108: 417–485 (1984).
Haring MA, Gao J, Volbeda T, Rommens CMT, Nijkamp HJJ, Hille J: A comparative study of Tam3 and Ac transposition in transgenic tobacco and petunia plants. Plant Mol Biol 13: 189–201 (1989).
Haring MA, Rommens CMT, Nijkamp HJJ, Hille J: The use of transgenic plants to understand transposition mechanisms and to develop transposon tagging strategies. Plant Mol Biol 16: 449–461 (1991).
Haring MA, Teeuwen-De Vroomen MJ, Nijkamp HJJ, Hille J:Trans-activation of an artificial dTam3 transposable element in transgenic tobacco plants. Plant Mol Biol 16: 39–48 (1991).
Hehl R, Baker B: Induced transposition ofDs by a stableAc in crosses of transgenic tobacco plants. Mol Gen Genet 217: 53–59 (1989).
Hehl R, Baker B: Properties of the maize transposable element Activator in transgenic tobacco plants: a versatile interspecies genetic tool. Plant Cell 2: 709–721 (1990).
Hehl R, Nacken WKF, Krause A, Saedler H, Sommer H: Structural analysis of Tam3, a transposable element fromAntirrhinum majus, reveals homologies to the Ac element from maize. Plant Mol Biol 16: 369–371 (1991).
Hudson AD, Carpenter R, Coen ES: Phenotypic effects of short range and aberrant transposition inAntirrhinum majus. Plant Mol Biol 14: 835–844 (1990).
Jones JDG, Carland F, Lim E, Ralston E, Dooner H: Preferential transposition of the maize element Activator (Ac) to linked chromosomal locations in tobacco. Plant Cell 2: 701–707 (1990).
Kaufmann PD, Doll RF, Rio DC:Drosophila P element transposase recognizes internal P element sequences. Cell 59: 359–371 (1989).
Kunze R, Starlinger P: The putative transposase of transposable element Ac fromZea mays L. interacts with subterminal sequences of Ac. EMBO J 8: 3177–3185 (1989).
Martin C, Carpenter R, Sommer H, Saedler H, Coen EC: Molecular analysis of instability in flower pigmentation ofAntirrhinum majus, following isolation of thepallida locus by transposon tagging. EMBO J 4: 1625–1630 (1985).
Martin C, Prescott A, Lister C, MacKay S: Activity of the transposon Tam3 inAntirrhinum and tobacco: possible role of DNA methylation. EMBO J 8: 997–1004 (1989).
Masson P, Federoff N: Mobility of the maizeSuppressor-mutator element in transgenic tobacco. Proc Natl Acad Sci USA 86: 2219–2223 (1989).
Masson P, Strem M, Federoff N: ThetnpA andtnpD gene products of the Spm element are required for transposition in tobacco. Plant Cell 3: 73–85 (1991).
O'Hare K, Rubin GM: Structures of P-transposable elements and their sites of insertion and excision in theDrosophila melanogaster genome. Cell 34: 25–35 (1983).
Pereira A, Saedler H: Transpositional behavior of the maize En/Spn element in transgenic tobacco. EMBO J 8: 1315–1321 (1989).
Robbins TP, Carpenter R, Coen ES: A chromosome rearrangement suggests that donor and recipient sites are associated during Tam3 transposition inAntirrhinum majus. EMBO J 8: 5–13 (1989).
Saedler H, Nevers P: Transposition in plants: a molecular model. EMBO J 4: 585–590 (1985).
Saiki RK, Gelfand DH, Stoffel S, Schraf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA: primer directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239: 487–491 (1988).
Sanger F, Nicklen S, Coulson R: DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1980).
Searles LL, Greenleaf AL, Kemp WE, Voelker RA: Sites of P-element insertion and structures of P-element deletions in the 5′ region of theDrosophila melanogaster RpII215. Mol Cell Biol 6: 3312–3319 (1986).
Sommer H, Bonas U, Saedler H: Transposon-induced alterations in the promoter region affect transcription of the chalcone synthase gene ofAntirrhinum majus. Mol Gen Genet 211: 49–55 (1988).
Sundaresan V, Freeling M: An extrachromosonal form of the Mu transposons in maize. Proc Natl Acad Sci USA: 84: 4924–4928 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Haring, M.A., Scofield, S., Teeuwen-de Vroomen, M.J. et al. Novel DNA structures resulting from dTam3 excision in tobacco. Plant Mol Biol 17, 995–1004 (1991). https://doi.org/10.1007/BF00037139
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00037139