Abstract
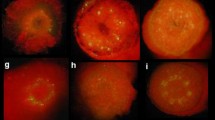
A transformation system was established for red raspberry, blackberry and blackberry x raspberry hybrids, utilizing the binary vector system of Agrobacterium tumefaciens. Leaf discs or internodal stem segments were inoculated with Agrobacterium strain LBA4404 containing the binary vectors PBI121.X, which has the β-glucuronidase (GUS) marker gene, or Bin 19, which has the neomycin phosphotransferase II (NPT II) gene. Regenerants were produced on media containing MS salts, 20 gl-1 sucrose, 7 gl-1 agar, 100 mgl-1 inositol, 0.5 mgl-1 nicotinic acid, 0.5 mgl-1 pyridoxine-HCl, 0.1 mgl-1 thiamine, and either 0.1 mgl-1 IBA and 2 mgl-1 BAP for leaf discs, or 0.2 mgl-1 BAP and 0.2 mgl-1 2,4-D for stem segments. Kanamycin sulphate, which was used as a selective agent for the NPT II gene, inhibited organogenesis at 50 mgl-1 and was therefore unsuitable for use as a selectable marker gene in Rubus. All regenerants were assayed utilizing the fluorogenic assay procedure to determine if the GUS gene had been transferred into the material and could therefore cleave the substrate 4-methyl-umbelliferyl-β-D-glucuronide. Seven GUS-positive plantlets were obtained which confirmed that this marker gene had been transferred into Rubus. A ‘dot blot’ assay was carried out on GUS-positive plant material to establish if the NPT II gene had also been transferred to the plant material.
Similar content being viewed by others
References
Bevan M (1984) Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res 12: 8711–8721
Fraley RT, Rogers SG, Horsch RB, Sanders PR, Flick JS, Adams SP, Bittner ML, Brand LA, Fink CL, Fry JS, Galluppi GR, Goldberg SB, Hoffmann NL, Woo SC (1983) Expression of bacterial genes in plant cells. Proc Nat Acad Sci USA 80: 4803–4807
Fillatti JAJ, Sellmer J, McCown B, Haissig B, Comai L (1987) Agrobacterium mediated transformation and regeneration of Populus. Mol Gen Genet 206: 192–199
Guerche P, Jouanin L, Tepfer D, Pelletier G (1987) Genetic transformation of oilseed rape (Brassica napus) by the Ri T-DNA of Agrobacterium rhizogenes and analysis of inheritance of the transformed phenotype. Mol Gen Genet 206: 382–386
Herrera-Estrella L, De Block M, Messens E, Hernalsteens J-P, Van Montagu M, Schell J (1983) Chimeric genes as dominant selectable markers in plant cells. EMBO J 2 987–995
Hille J, Klasen I, Schilperoort R (1982) Construction and application of R prime plasmids, carrying different segments of an octopine Ti plasmid from A. tumefaciens, for complementation of vir genes. Plasmid 7: 107–118
Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA (1983) A binary plant vector strategy based on separation of vir and T-region of the A. tumefaciens Ti plasmid. Nature 303: 179–180
Horsch RB, Klee HJ (1986) Rapid assay of foreign gene expression in leaf discs transformed by A. tumefaciens: role of T-DNA borders in transfer process. Proc Nat Acad Sci USA 83: 4428–4432
James DJ (1987) In: Russel GE (Ed) Biotechnology and Genetic Engineering Reviews. Vol 5 (pp 33–79)Intercept, Newcastle-upon-Tyne
James DJ, Passey AJ (1989) Genetic transformation of apple (Malus pumila Mill.) using a disarmed Ti binary vector. Plant Cell Rep (in press)
Jefferson RA (1987) Assaying chimeric genes in plants: The GUS gene fusion system. Plant Mol Biol Reporter 8: 387–405
Jefferson RA (1987) GUS Gene Fusion System Users Manual. Version 1.21. Department of Molecular Genetics, Plant Breeding Institute, Cambridge, England
Joos H, Inze D (1983) Genetic analysis of T-DNA transcripts in nopaline crown galls. Cell 32: 1057–1067
Lemmers M, De Beuckeleer M, Holsters M, Zambryski P, Depicker A, Hernalsteens JP, Van Montagu M, Schell J (1980) Internal organisation boundaries and integration of Ti-plasmid DNA in nopaline crown gall tumours. J Mol Biol 144: 353–376
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular Cloning. A Laboratory Manual. Cold Spring Harbour Laboratory
Muller AJ, Mendel RR, Schiemann J, Simoens C, Inze D (1987) High meiotic stability of a foreign gene introduced into tobacco by Agrobacterium-mediated transformation. Mol Gen Genet 207: 171–175
McDonnell RE, Clark RD, Smith WA, Hinchee MA (1987) A simplified method for the detection of neomycin phosphotransferase II activity in transformed plant tissue. Plant Mol Biol Reporter 5: 380–386
McNicol RJ, Graham J (1988) Agrobacterium spp. as vectors of DNA. Annual Report of Scottish Crop Research Institute for 1987 (pp89–90)
McNicol RJ, Graham J (1989) In vitro regeneration of Rubus from leaf and stem segments. Plant Cell Tissue Organ Culture (in press)
Paszkawski J, Shillito RD, Saul M, Mandak V, Hohn T, Hohn B, Potrykus I (1984) Direct gene transfer to plants. EMBO J 3: 2717–2722
Radke SE, Andrews BM, Moloney MM, Crouch ML, Kridl JC, Knauf VC (1988) Transformation of Brassica napus L. using Agrobacterium tumefaciens: developmentally regulated expression of a reintroduced napin gene. Theor Appl Genet 75: 685–694
Reiss B, Sprengel R, Will H, Schaller H (1984) A new sensitive method for qualitative and quantitative assay of neomycin phosphotransferase in crude cell extracts. Gene 30: 211–218
Simpson RB, Spielmann A, Margossian L, McKnight TD (1986) A disarmed binary vector for Agrobacterium tumefaciens functions in A. rhizogenes. Plant Mol Biol 6: 403–415
Zambryski P, Depicker A, Kruger D, Goodman HM (1982) Tumour induction by Agrobacterium tumefaciens: analysis of the boundaries of T-DNA. J Mol Appl Genet 1: 361–370
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Graham, J., McNicol, R.J. & Kumar, A. Use of the GUS gene as a selectable marker for Agrobacterium-mediated transformation of Rubus . Plant Cell Tiss Organ Cult 20, 35–39 (1990). https://doi.org/10.1007/BF00034754
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00034754