Abstract
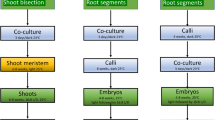
Agrobacterium rhizogenes transformed and control roots of the tetraploid potato cv. Bintje were compared. Transformed roots were obtained after infection by A. rhizogenes 15834 or 1855. Both in leaf and stem segments, more roots were formed at the basal side of the segments, indicative for a polarity in root formation. As compared to control roots the transformed roots are characterized by smaller and more densely stained cells, a zone of cell division, and smaller statoliths. These characteristics are correlated with vigorous growth, high branching incidence and diminished geotropism. The plant regeneration procedure according to Ooms et al. [1] was modified. The transformed roots required less 2,4-D than control roots for the induction of shoot-competent calli. The callus and shoot induction phases were reduced from 8 and 6 weeks to 3 and 3 weeks, respectively. Upon induction, 25%, 58% and 61% of the root clones originating from tuber, stem and leaf, respectively, produced shoots, whereas all of the control roots produced shoots. Shoot outgrowth occurred on liquid MS medium in the absence of hormones.
Similar content being viewed by others
Abbreviations
- Ri-root:
-
Agrobacterium rhizogenes transformed root
- BAP:
-
benzylaminopurine
- IAA:
-
indoleacetic acid
- GA3 :
-
gibberellic acid
- NAA:
-
naphthaleneacetic acid
- 2,4-D:
-
2,4 dichlorophenoxyacetic acid
References
Ooms G, Karp A, Burrell MM, Twell D, Roberts J (1985) Genetic modification of potato development using Ri T-DNA. Theor Appl Genet 70: 440–446
Petit A, David C, Ellis GA, Guyon JG, Casse-Delbart F, Tempé J (1983) Further extension of the opine concept: plasmids in Agrobacterium rhizogenes cooperate for opine degradation. Mol Gen Genet 190: 204–214
Huffman GA, White FF, Gordon MP, Nester EW (1984) Hairy root inducing plasmid: physical map and homology to tumor-inducing plasmids. J Bacteriol 157: 269–276
Jouanin L (1984) Restriction map of an agropine-type Ri-plasmid and its homologies with Ti plasmids. Plasmid 12: 91–102
White FF, Taylor BH, Huffman GA, Gordon MP, Nester EW (1985) Molecular and genetic analysis of the transferred DNA regions of the root-inducing plasmid of Agrobacterium rhizogenes. J Bacteriol 164: 33–44
Offringa IA, Melchers LS, Regensburg-Tuinck AJG, Costantino P, Schilperoort RA, Hooykaas PJJ (1986) Complementation of Agrobacterium tumefaciens tumor-inducing aux mutants by genes from the TR-region of the Ri plasmid of Agrobacterium rhizogenes. Proc Natl Acad Sci USA 83: 6935–6939
Vilaine F, Charbonnier C, Casse-Delbart F (1987) Further insight concerning the TL region of the Ri plasmid of Agrobacterium rhizogenes strain A4: transfer of a 1.9 kb fragment is sufficient to induce transformed roots on tobacco leaf fragments. Mol Gen Genet 210: 111–115
Spena A, Schmülling T, Koncz C, Schell JS (1987) Independent and synergistic activity of rol A,B and C loci in stimulating abnormal growth in plants. EMBO J 6: 3891–3899
Tepfer D (1984) Transformation of several species of higher plants by Agrobacterium rhizogenes: sexual transmission of the transformed genotype and phenotype. Cell 37: 959–967
David C, Tempé J (1988) Genetic transformation of cauliflower (Brassica oleracea L. var. Botrytis) by Agrobacterium rhizogenes. Plant Cell Rep 7: 88–91
Peterson RL (1975) The initiation and development of root buds. In: Torrey JG, Clarkson DT (Eds) The Development and Function of Roots (pp 125–161) Academic Press, London/New York/San Francisco
Hoekema A, Hooykaas PJ, Schilperoort RA (1984) Transfer of the octopine T-DNA segment to plant cells mediated by different types of Agrobacterium tumor or root-inducing plasmids: generalities of virulence systems. J Bacteriol 158: 383–385
Simpson RB, Spielmann A, Margossian L, McKnigt TD (1986) A disarmed binary vector from Agrobacterium tumefaciencs functions in Agrobacterium rhizogenes. Plant Mol Biol 6: 403–415
Sree Ramulu K (1986) Case histories of genetic variability in vitro: potato. In Vasil IK (Ed) Cell Culture and Somatic Cell Genetics, Vol 3 (pp 449–473) Academic Press, New York
Hänisch ten Cate ChH, Ennik E, Roest S, Sree Ramulu K, Dijkhuis P, de Groot B (1988) Regeneration and characterisation of plants from potato root lines transformed by Agrobacterium rhizogenes. Theor Appl Genet 75: 452–459
Ottaviani MP, Hänisch ten Cate ChH (1987) Plant regeneration from normal and Agrobacterium rhizogenes-transformed roots in the tetraploid potato cultivar Bintje. Acta Bot Neerl 36 (Suppl 1): 45
Anand VK, Haberlein GT (1977) Crown gall tumorigenesis ii potato tuber tissue. Am J Bot 64: 153–158
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissues cultures. Physiol Plant 15: 473–497
Hänisch ten Cate ChH, Sree Ramulu K, Dijkhuis P, de Groot B (1987) Genetic stability of cultured hairy roots induced by Agrobacterium rhizogenes on tuber discs of potato cv. Bintje. Plant Sci 49: 217–222
Gerrits PO, Smid L (1983) A new, less toxic polymerization system for the embedding of soft tissues in glycol methylacrylate and subsequent preparing of serial sections. J Microsc 132: 81–85
Jensen W (1962) Botanical Histochemistry: Principles and Practice. WH Freeman, San Fransisco/London
Chriqui D, David C, Adam S (1988) Effect of differentiated or dedifferentiated state of tobacco pith tissue on its behaviour after inoculation with Agrobacterium rhizogenes. Plant Cell Rep 7: 111–114
Cardarelli M, Spano L, De Paolis A, Mauro ML, Vitali G, Costantino P (1985) Identification of the genetic locus responsible for non polar induction by Agrobacterium rhizogenes 1855. Plant Mol Biol 5: 385–391
Ryder MH, Tate ME, Kerr A (1985) Virulence properties of strains of Agrobacterium on the apical and basal surfaces of carrot root discs. Plant Physiol 77: 215–221
Jouanin L, Guerche P, Pamboukdjian N, Tourneur C, Casse-Delbart F, Tourneur J (1987) Structure of T-DNA in plants regenerated from roots transformed by Agrobacterium rhizogenes strain A4. Mol Gen Genet 206: 389–392
Byrne MC, Koplow J, David C, Chilton MD (1983) Structure of T-DNA in roots transformed by Agrobacterium rhizogenes. J Mol Appl Genet 2: 201–209
Schmülling T, Schell J, Spena A (1988) Single genes from Agrobacterium rhizogenes influence plant development. EMBO J 7: 2621–2629
Loomis RS, Torrey JG (1964) Chemical control of vascular cambium initiation in isolated radish roots. Proc Natl Acad Sci USA 52: 3–11
Noll F (1892) Ueber Heterogene Induktion. Leipzig
Volkmann D, Sievers (1979) Graviperception in multicellular organs. In: Haupt W, Feinleib M (Eds) Physiology of Movements. Encyclopaedia Plant Physiology, NS 7: 573–600
Van de Geyn SC, Helder J, van Hooren HG, Hänisch ten Cate ChH (1988) Growth, root respiration and phosphorous utilization of normal and Agrobacterium rhizogenes transformed potato plants. Plant Soil 111: 283–288
Ambros PF, Matzke AJM, Matzke MA (1986) Localization of Agrobacterium rhizogenes T-DNA in plant chromosomes by in situ hybridization. EMBO J 5: 2073–2077
Ooms G, Twell D, Bossen ME, Hage JC, Burrell MM (1986) Developmental regulation of Ri TL-DNA gene expression in roots, shoots and tubers of transformed potato (Solanum tuberosum cv. Désirée). Plant Mol Biol 6: 321–330
Trulson AJ, Simpson RB, Shahin AE (1986) Transformation of cucumber (Cucumis sativus L.) plants with Agrobacterium rhizogenes. Theor Appl Genet 73: 11–15
Shen WH, Petit A, Guern J, Tempé J (1988) Hairy roots are more sensitive to auxin than normal roots. Proc Natl Acad Sci USA 85: 3417–3421
Canfield ML, Moore LW (1983) Production of ethylene by Daucus carota inoculated with Agrobacterium tumefaciens and Agrobacterium rhizogenes. Z Pflanzenphysiol 112: 471–474
Minota T, Kikuta Y, Okazawa Y (1979) Effect of ethylene on sprout growth and endogenous growth substances of potato plants. J Fac Agric Hokkaido Univ 59: 239–248
Maan AC, van der Linde PCG, Harkes PAA, Libbenga KR (1985) Correlation between the presence of membrane-bound auxin binding and root regeneration in cultured tobacco-cells. Planta 164: 376–378
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ottaviani, M.P., Schel, J.H.N. & Hänisch ten Cate, C.H. Variation in structure and plant regeneration of Agrobacterium rhizogenes transformed and control roots of the potato cv. Bintje. Plant Cell Tiss Organ Cult 20, 25–34 (1990). https://doi.org/10.1007/BF00034753
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00034753